1,4-naphthoquinone compounds and application thereof
A compound, naphthoquinone technology, applied in 1 field, can solve the problems of limiting clinical application, toxicity and water solubility, etc., and achieve significant anti-tumor activity
Inactive Publication Date: 2010-09-22
GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
View PDF0 Cites 3 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Shikonin compounds are a class of natural naphthoquinone compounds that widely exist in nature and have significant antitumor activity, but their clinical application is limited due to the toxicity and poor water solubility of these compounds
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
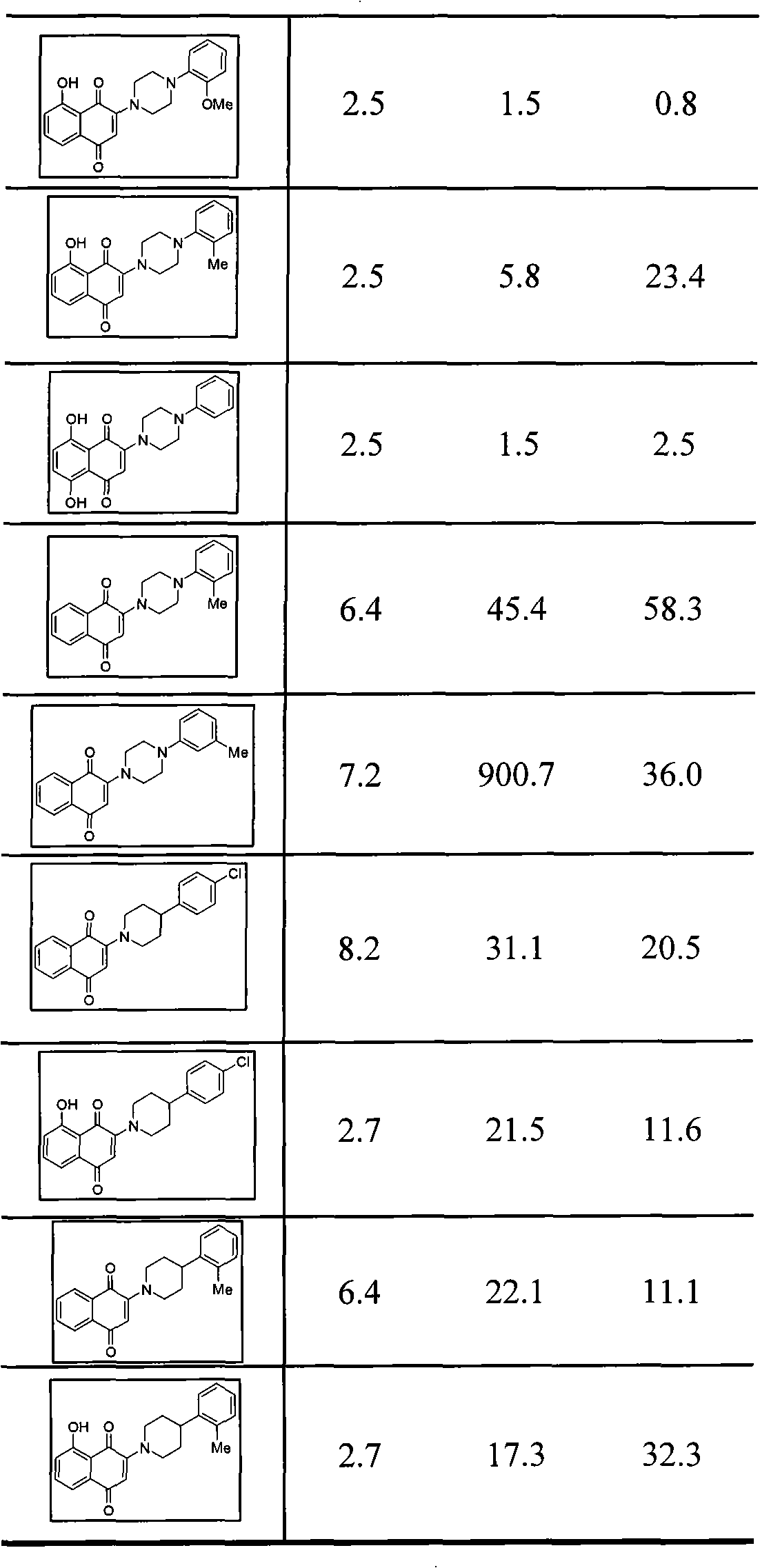
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory concentration | aaaaa | aaaaa |
Login to View More
Abstract
The invention provides 1,4-naphthoquinone compounds with a formula II, and the definition of substituents of the naphthoquinone compounds are described as the specification. The compounds have the connecting ring structures of piperazine or piperidine, have notable antitumor activity, and provide a new choice for preparing antitumor drugs in clinical practice.
Description
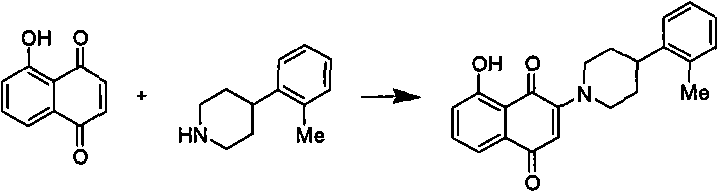
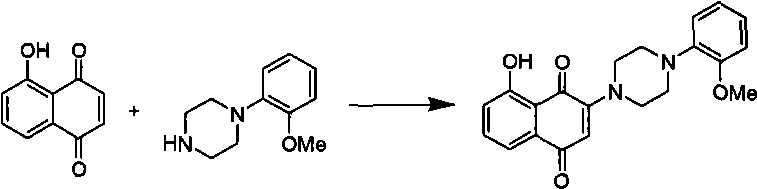
technical field The invention relates to a class of 1,4-naphthoquinone compounds and applications thereof. Background technique Worldwide, the cost of new drug research and development is increasing year by year, but the number of new drugs on the market is decreasing, and the efficiency of new drug research and development is decreasing year by year. In recent years, the number of new drugs approved by countries around the world has shown a new "trough" in the past 20 years. The process of drug innovation is very complicated. First, the development cycle of new drugs is long. It usually takes 10 to 15 years from the synthesis of active compounds to the launch of drugs. Second, the investment in new drug research and development is large. In the United States, it takes about 8 Thirdly, the success rate of new drug research and development is low. Of the 10,000 compounds with in vitro activity synthesized, only 250 can enter animal experiments, only 5 can enter clinical tri...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D295/116C07D211/14C07D211/18A61K31/495A61K31/451A61P35/00
Inventor 丁克裴端卿周净段磊
Owner GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Features
- Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com