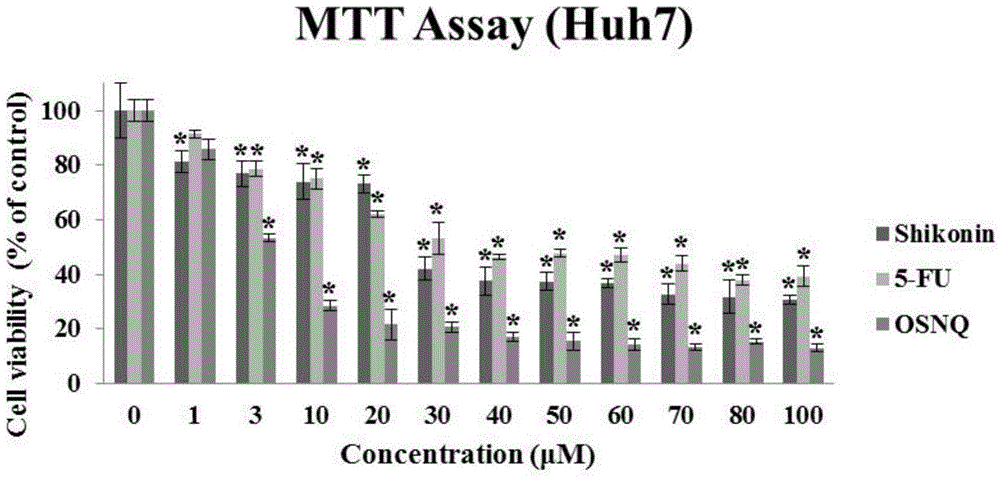
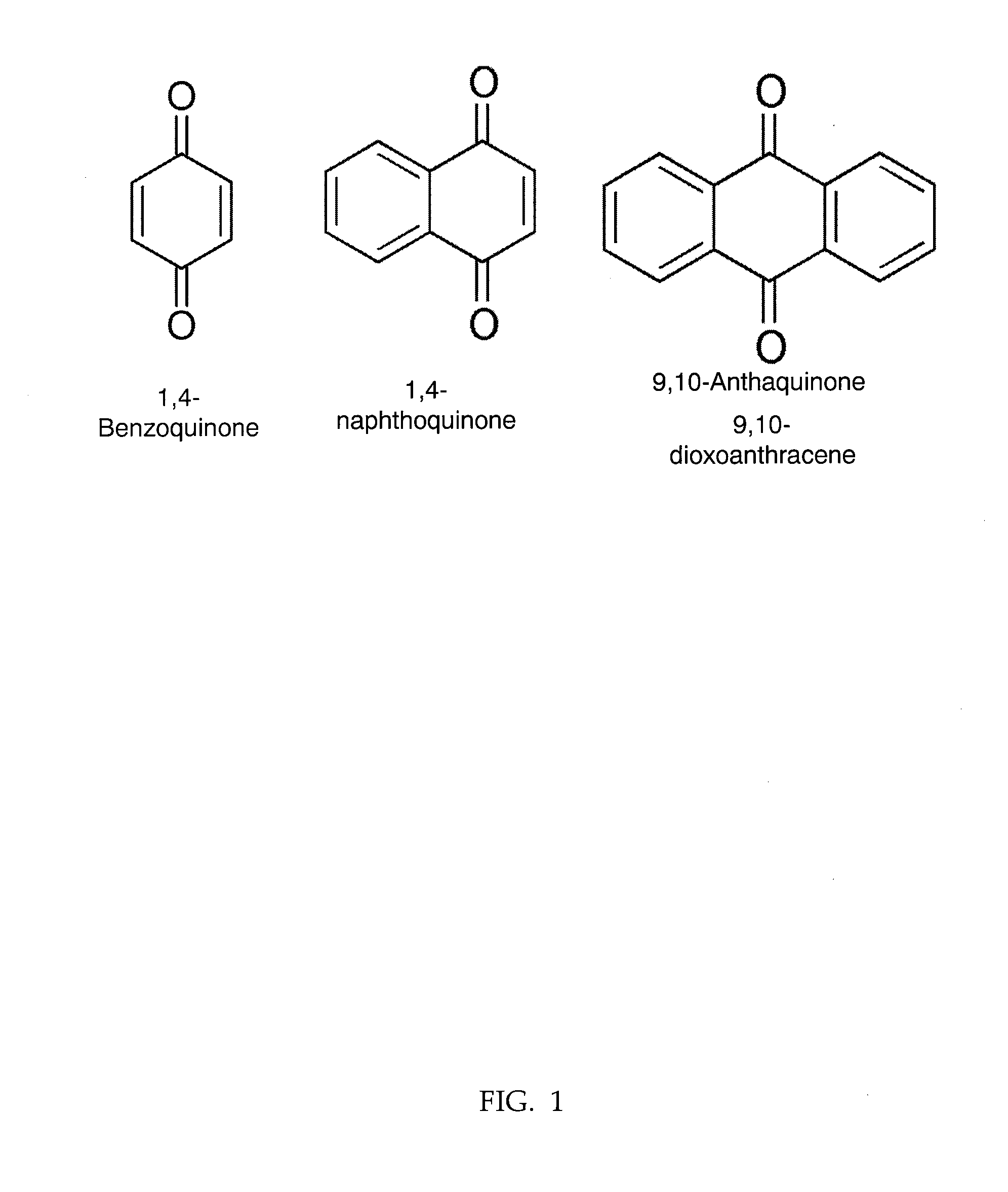
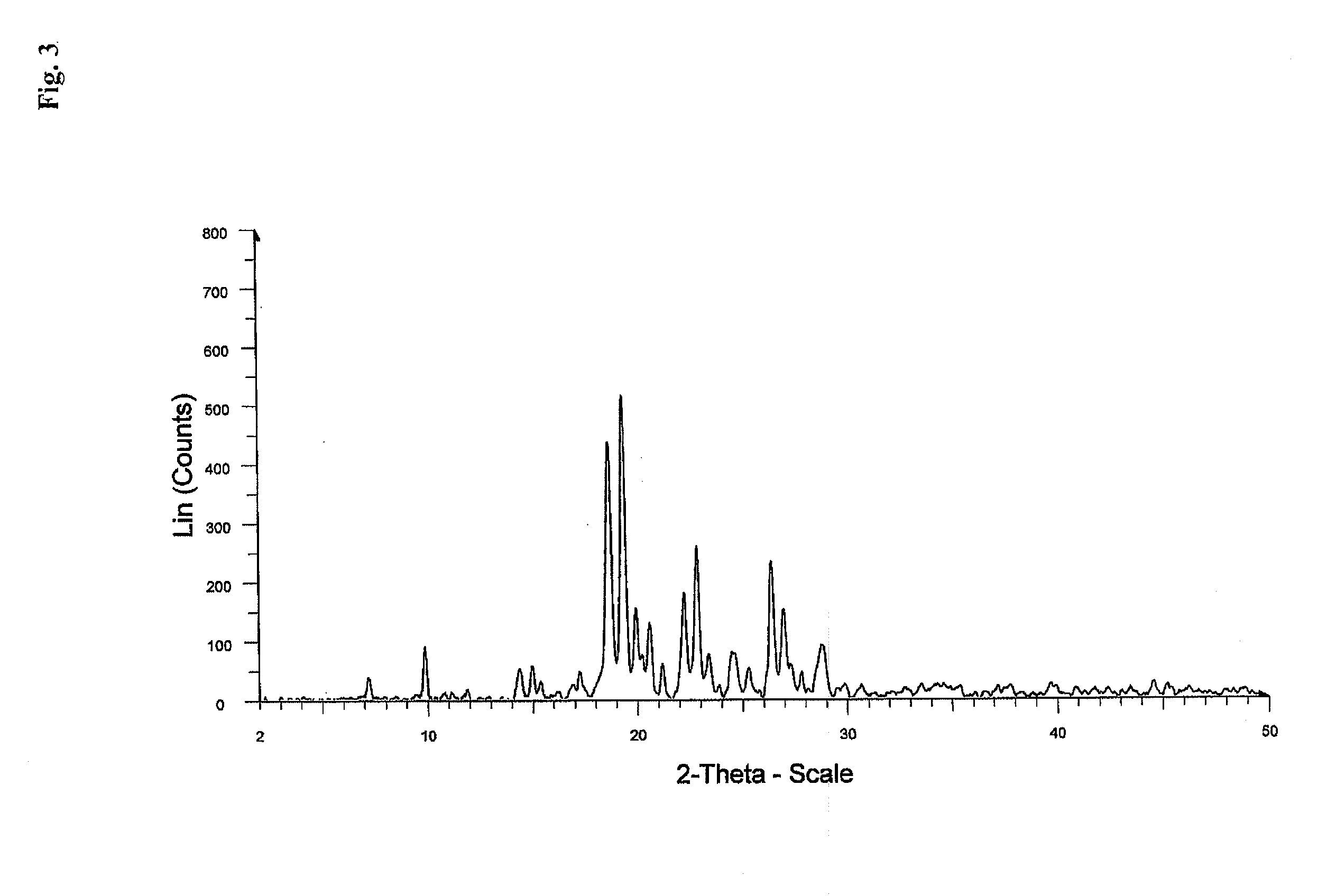
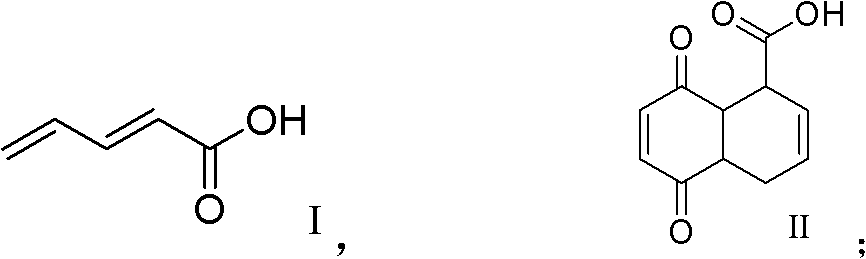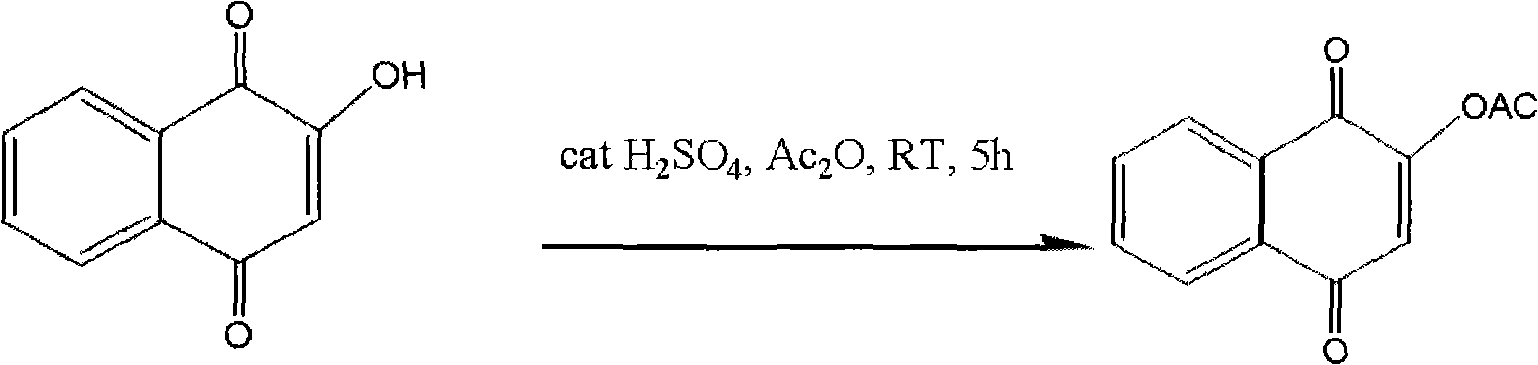Patents
Literature
81 results about "1,4-Naphthoquinone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
1,4-Naphthoquinone or para-naphthoquinone is an organic compound derived from naphthalene. It forms volatile yellow triclinic crystals and has a sharp odor similar to benzoquinone. It is almost insoluble in cold water, slightly soluble in petroleum ether, and more soluble in polar organic solvents. In alkaline solutions it produces a reddish-brown color. Vitamin K is a derivative of 1,4-naphthoquinone. It is a planar molecule with one aromatic ring fused to a quinone subunit. It is an isomer of 1,2-naphthoquinone.
Cyanide-free silver plating solution additive
The invention relates to a cyanide-free silver plating solution additive which comprises the following components by ratio: 0.1-10g / l of brightener, 5-10g / l of leveling agent, 100-600g / l of complexing agent and the balance of plasma water, wherein the brightener is one or mixture of more in nitrogen-containing compound, triazole, benzotriazole, 2-hydroxypyridine, pyridine, 22 dipyridyl, 1, 10-phenanthroline, triethylene tetramine and diethylene triamine according to any ratio; the leveling agent is one or mixture of more in aromatic hydrocarbon compounds, naphthalene, 1-methylnaphthalene, 1, 4-naphthoquinone and 1-naphthol according to any ratio; the complexing agent is one or mixture of more in disodium ethylenediamine tetraacetate, niacin, aminosulfonic acid and potassium pyrophosphate according to any ratio. The cyanide-free silver plating solution additive has the beneficial effects that the plating solution is stable, low in toxicity and good in dispersing ability; the obtained plating layer is bright and fine as well as good in binding force; the technology adopts the environment-friendly organic additive which does not contain heavy metal and sulfide; the plating layer is good in corrosion resistance. Furthermore, the cyanide-free silver plating solution additive can be directly used for parts such as brass, copper, chemical nickel and the like, preplating is not needed, and the binding force is also guaranteed.
Owner:HANGZHOU WIN WIN TECH CO LTD
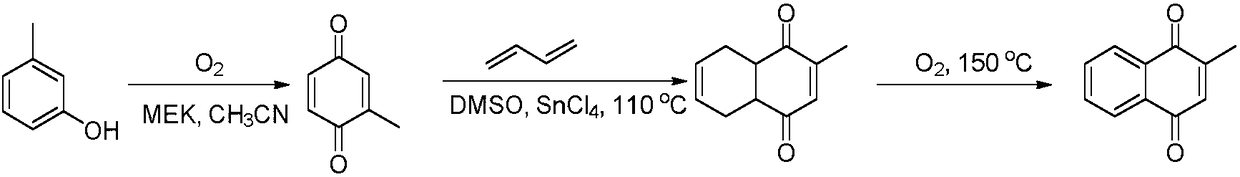
Process for preparation of 2-Methyl-1,4-naphthoquinone
InactiveUS6579994B2Low costReduce usageOrganic compound preparationQuinone preparation by oxidationAcetic acidNaphthoquinone
The present invention describes a process for the preparation of 2-Methyl-1,4-naphthoquinone by oxidizing 2-methylnaphthalene with hydrogen peroxide in the presence of acetic acid.
Owner:COUNCIL OF SCI & IND RES
Novel synthesis method of 2-methyl-1,4-naphthoquinone
InactiveCN103833541AReduce consumptionEasy to separateQuinone preparation by oxidationSynthesis methodsDistillation
The invention relates to a novel synthesis method of 2-methyl-1,4-naphthoquinone. The novel synthesis method comprises the steps: respectively dissolving a defined amount of 2-methylnaphthalene and metachloroperbenzoic acid in moderate glacial acetic acid to prepare solutions (1) and (2), dropwise adding the solution (2) in the solution (1) under the stirring, after reacting for a period of time at a certain temperature, extracting a reaction solution by using chloroform, washing an extraction solution with a saturated NaHCO3 solution and water, after anhydrous Na2SO4 is dried, performing reduced pressure distillation to prepare a crude product of the 2-methyl-1,4-naphthoquinone, and recrystalizing by using alcohol as a solvent to obtain a pure product of the 2-methyl-1,4-naphthoquinone. According to the novel synthesis method, the conversion rate of the 2-methylnaphthalene can reach 80 percent, and the yield of the 2-methyl-1,4-naphthoquinone can reach 31 percent. The method disclosed by the invention has the characteristics of short reaction time, mild conditions, and the like, and products are easily separated; and no any catalyst is used in the reaction, thus the energy consumption and the environment pollution can be reduced.
Owner:QILU UNIV OF TECH
Non-aqueous electrolyte for primary battery, and non-aqueous electrolyte primary battery using the same
InactiveUS20100129723A1Stable jobReduce voltage droopCell electrodesOrganic electrolyte cellsHigh rate1,4-Benzoquinone
It is an object to provide a non-aqueous electrolyte for use in a primary battery, which is advantageous in that the primary battery using the non-aqueous electrolyte suffers less lowering of voltage during the high-rate discharge and can work stably. Disclosed are a non-aqueous electrolyte for use in a primary battery, comprising at least one organic compound selected from the group consisting of 1,2-benzoquinone, 1,4-naphthoquinone, 1,2-naphthoquinone, 9,10-anthraquinone, 1,4-anthraquinone, acenaphthenequinone, and a derivative of 1,2-benzoquinone, 1,4-naphthoquinone, 1,2-naphthoquinone, 9,10-anthraquinone, 1,4-anthraquinone, acenaphthenequinone, or 1,4-benzoquinone; a non-aqueous electrolyte for use in a primary battery, comprising an organic compound exhibiting a reduction potential of 2.5 V or higher and a quantity of electricity in reduction reaction per electrode area of 1,000 mC / cm2 or lower under specific conditions; and a non-aqueous electrolyte for use in a primary battery, having a reduction potential of 2.5 V or higher and a quantity of electricity in reduction reaction per electrode area of 1,000 mC / cm2 or lower.
Owner:MITSUBISHI CHEM CORP
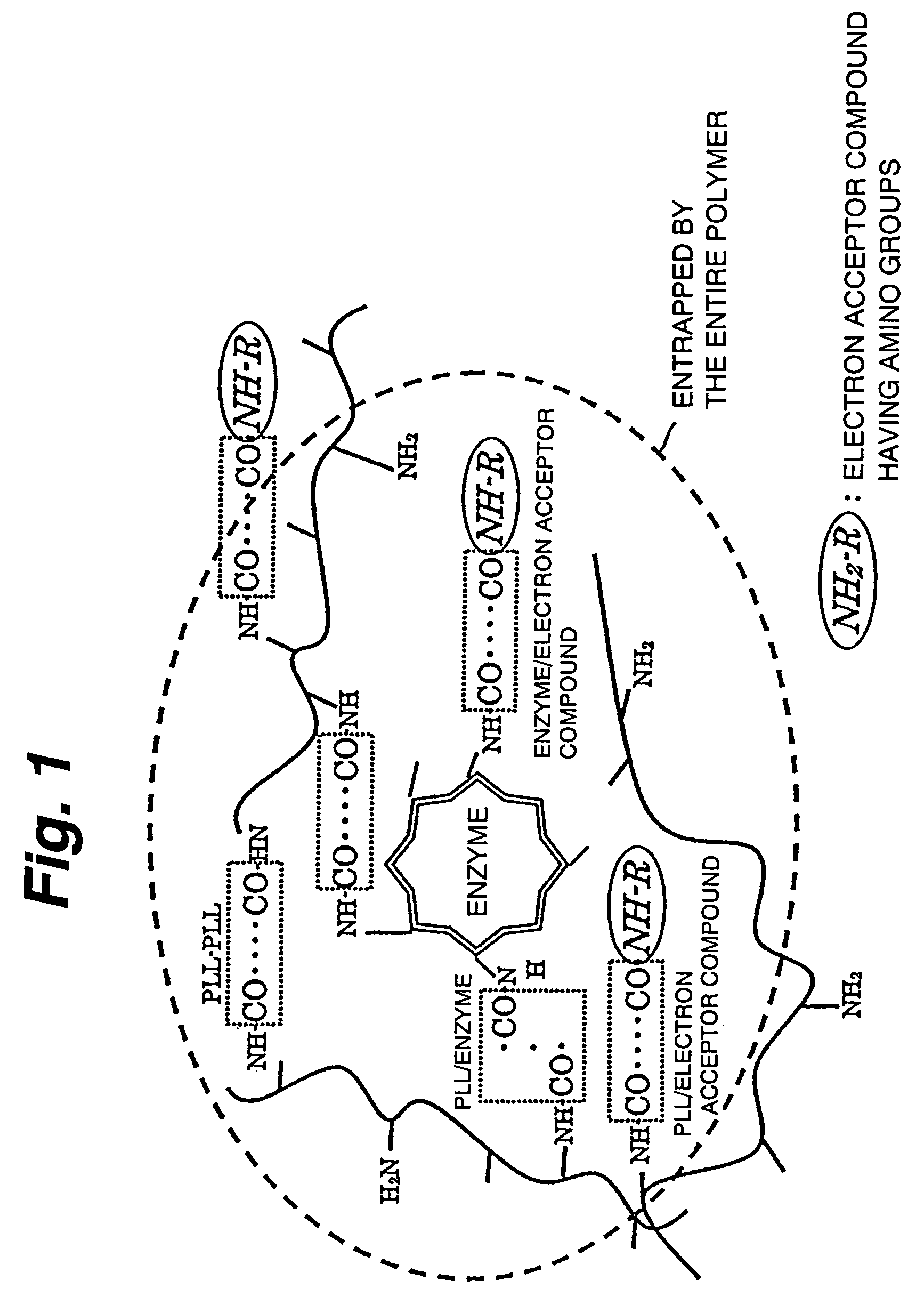
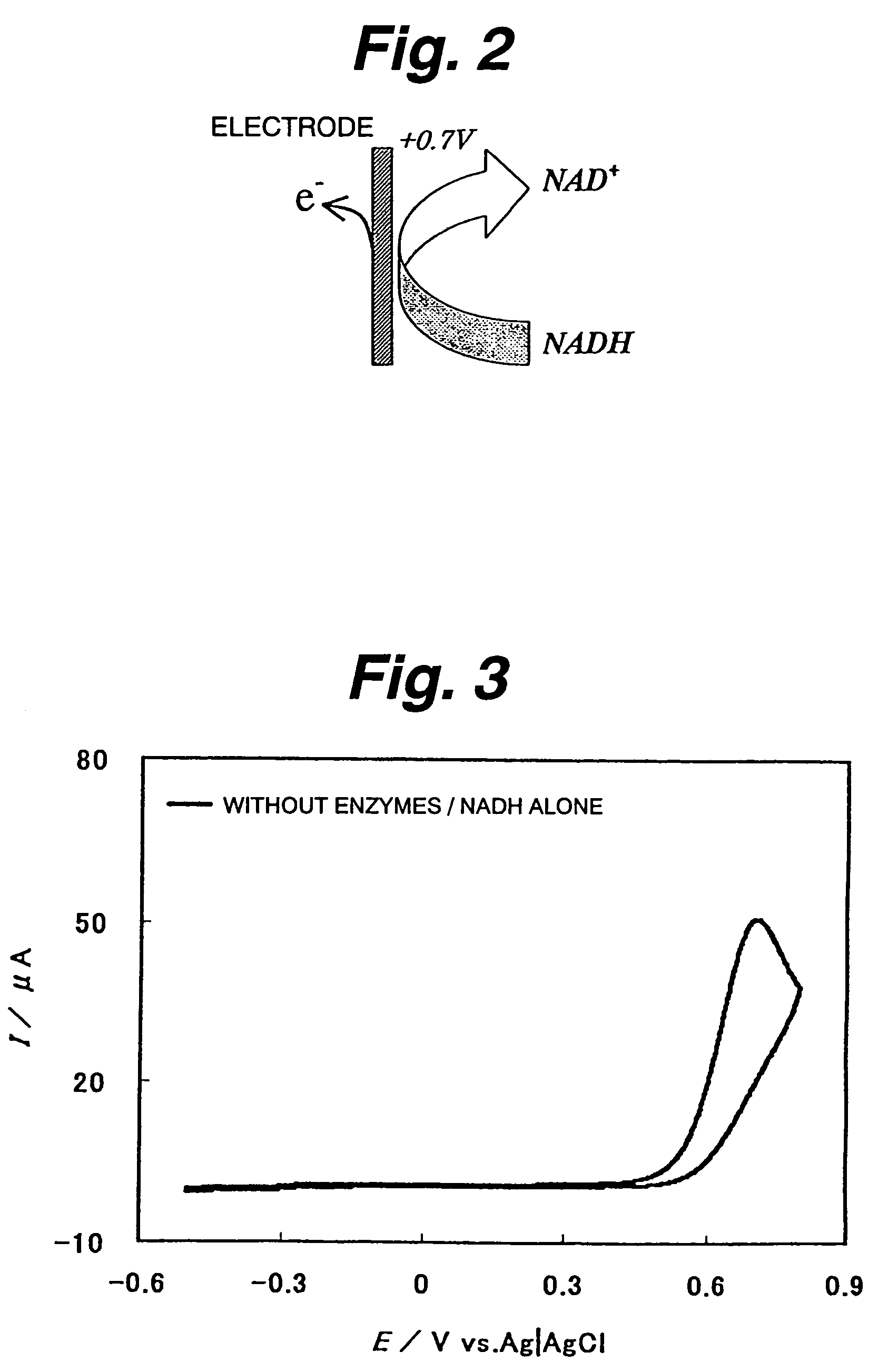
Immobilization support, process for producing the same, electrode, process for producing the same, electrode reaction utilizing apparatus and process for producing the same
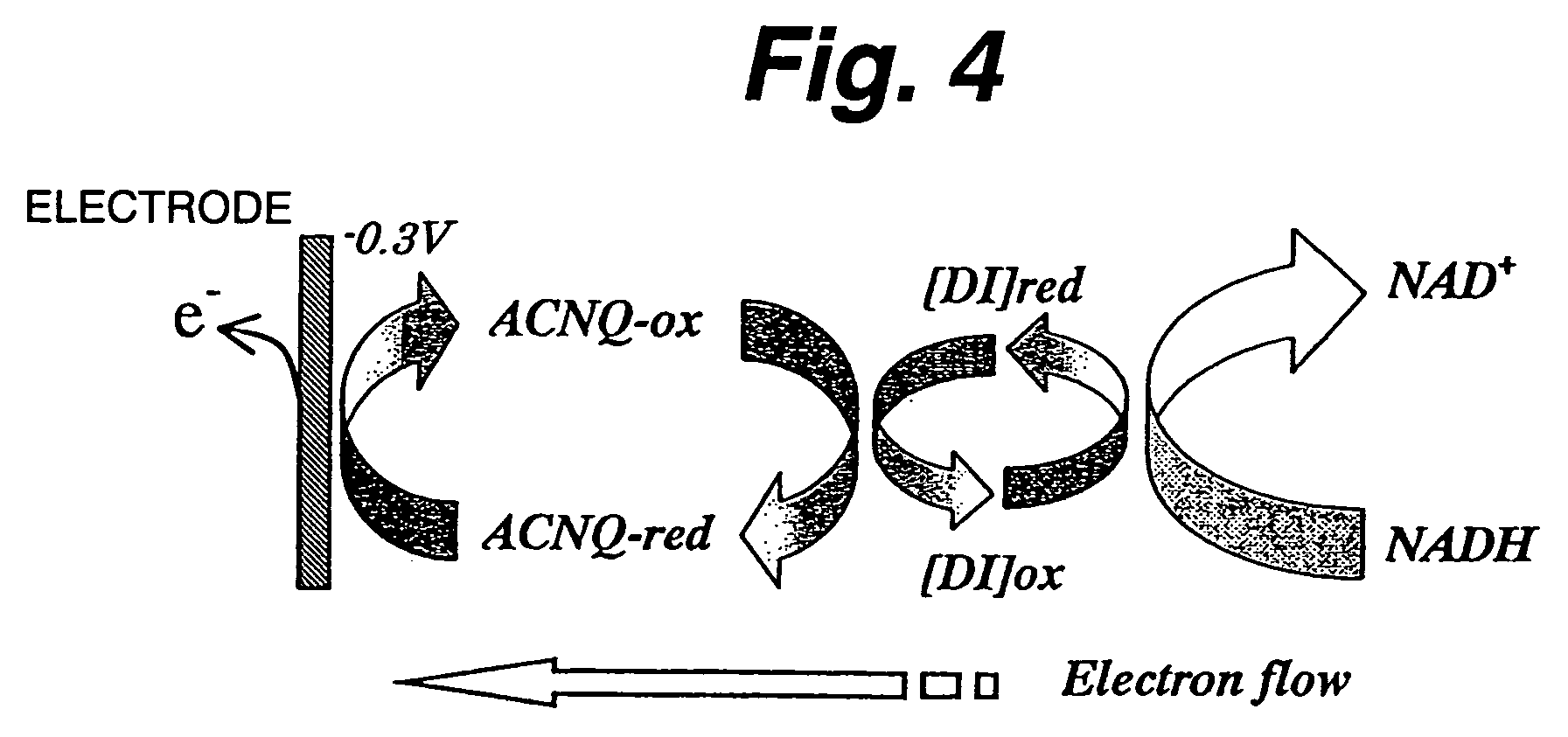
ActiveUS7520970B2Easy to useImprove performanceImmobilised enzymesBioreactor/fermenter combinationsNaphthoquinoneDiaphorase
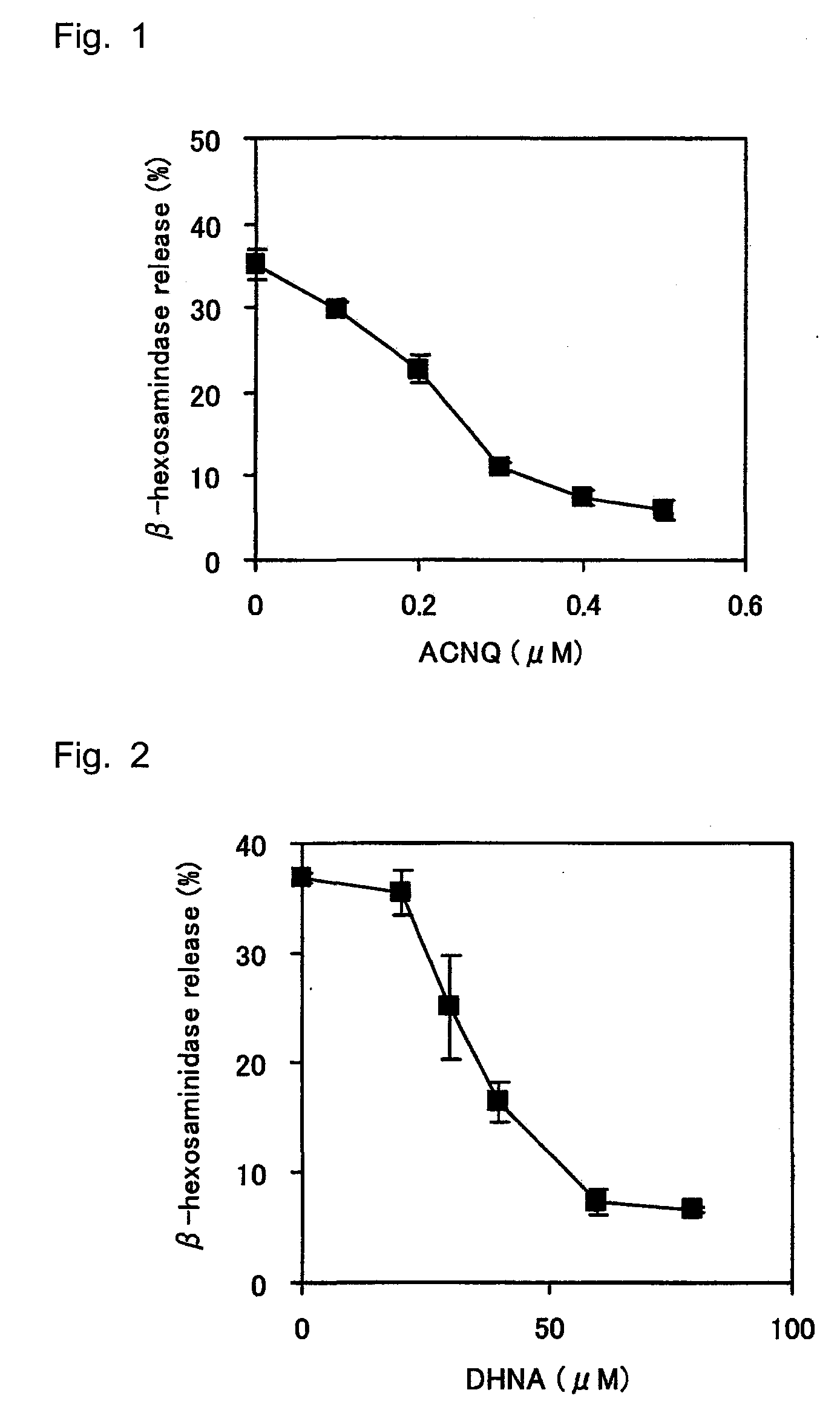
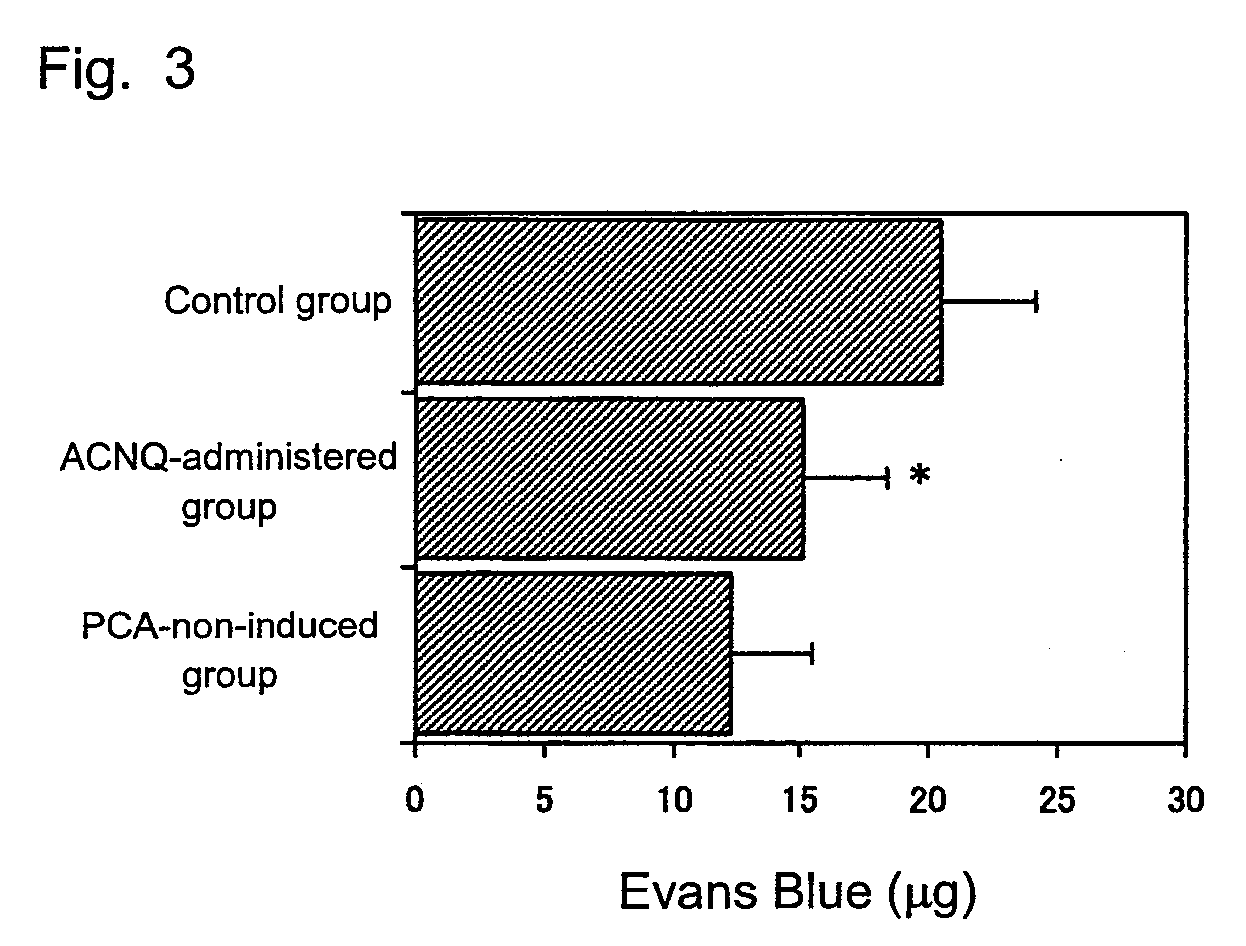
An immobilization carrier containing an electron acceptor compound is used in addition to glutaraldehyde and poly-L-lysine to immobilize an enzyme and an electron acceptor compound simultaneously to an electrode. For example, here are used diaphorase as the enzyme and 2-amino-3-carboxy-1,4-naphthoquinone (ACNQ) as the electron acceptor compound.
Owner:MURATA MFG CO LTD
Bi-component coating unsaturated polyester resin and preparation method thereof
The invention relates to a preparation method of a bi-component coating unsaturated polyester resin. The preparation method comprises the following steps of putting reactants and a first polymerization inhibitor accounting for 250 ppm-320 ppm of a total mass of the resin into a container to carry out polycondensation and dehydration reaction, wherein the reactants comprise a dihydric alcohol, allyl ether and unsaturated dicarboxylic acid or anhydride; cooling to a temperature of 120 DEG C-140 DEG C; adding the first polymerization inhibitor accounting for 40 ppm-80 ppm of the total mass of the resin; diluting by adding styrene; cooling to the temperature of 40-60 DEG C; and adding a second polymerization inhibitor accounting for 40 ppm-80 ppm of the total mass of the resin, wherein the second polymerization inhibitor is one or two of 1,4-naphthoquinone and p-benzoquinone. The bi-component coating unsaturated polyester resin prepared by the preparation method has a long resin activation period after an initiator is added; a drying time after the resin added with an accelerant and a resin added with the initiator are mixed is relatively short; and film-forming quality is high.
Owner:CHANGZHOU HUARI NEW MATERIAL
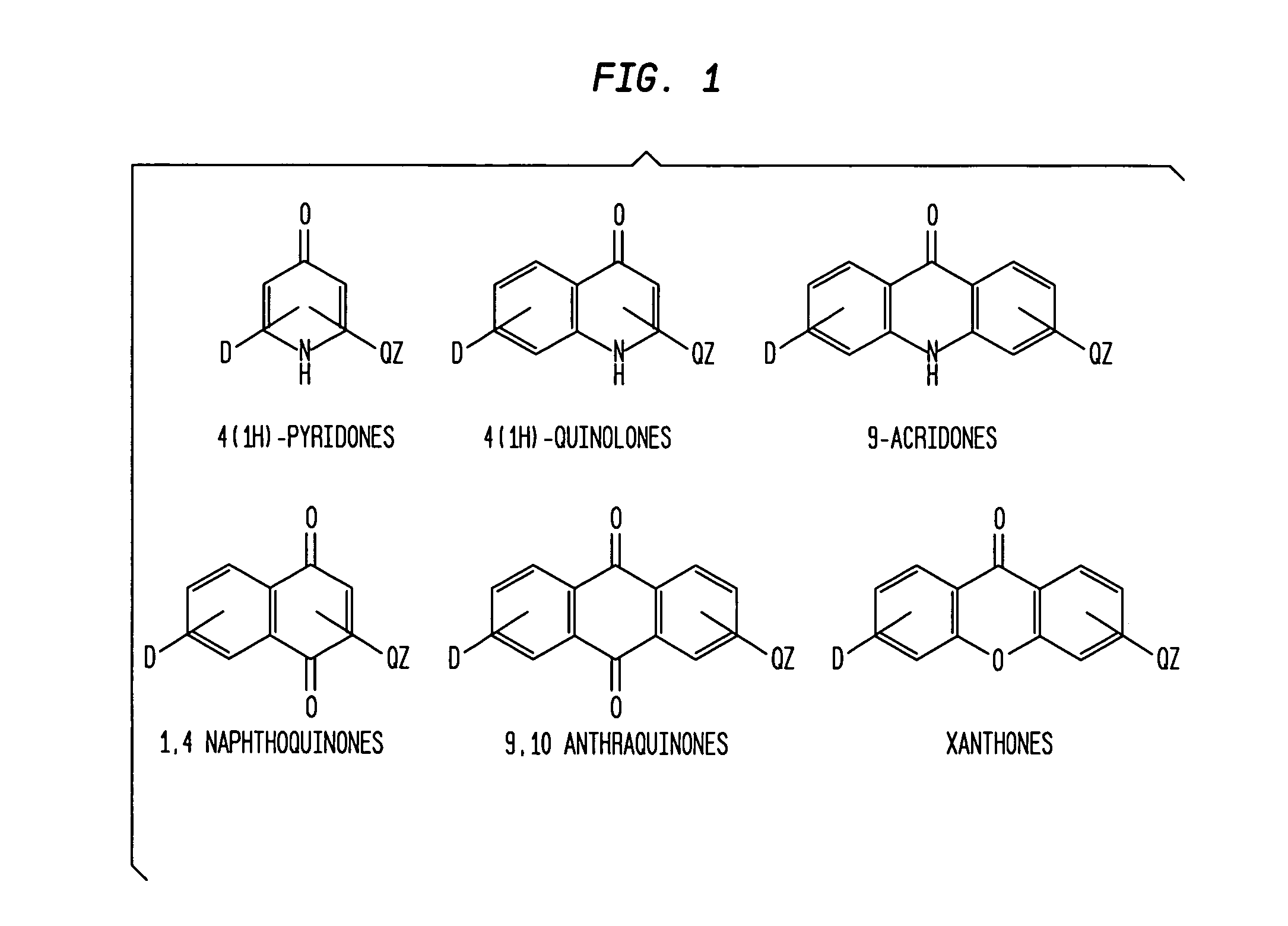
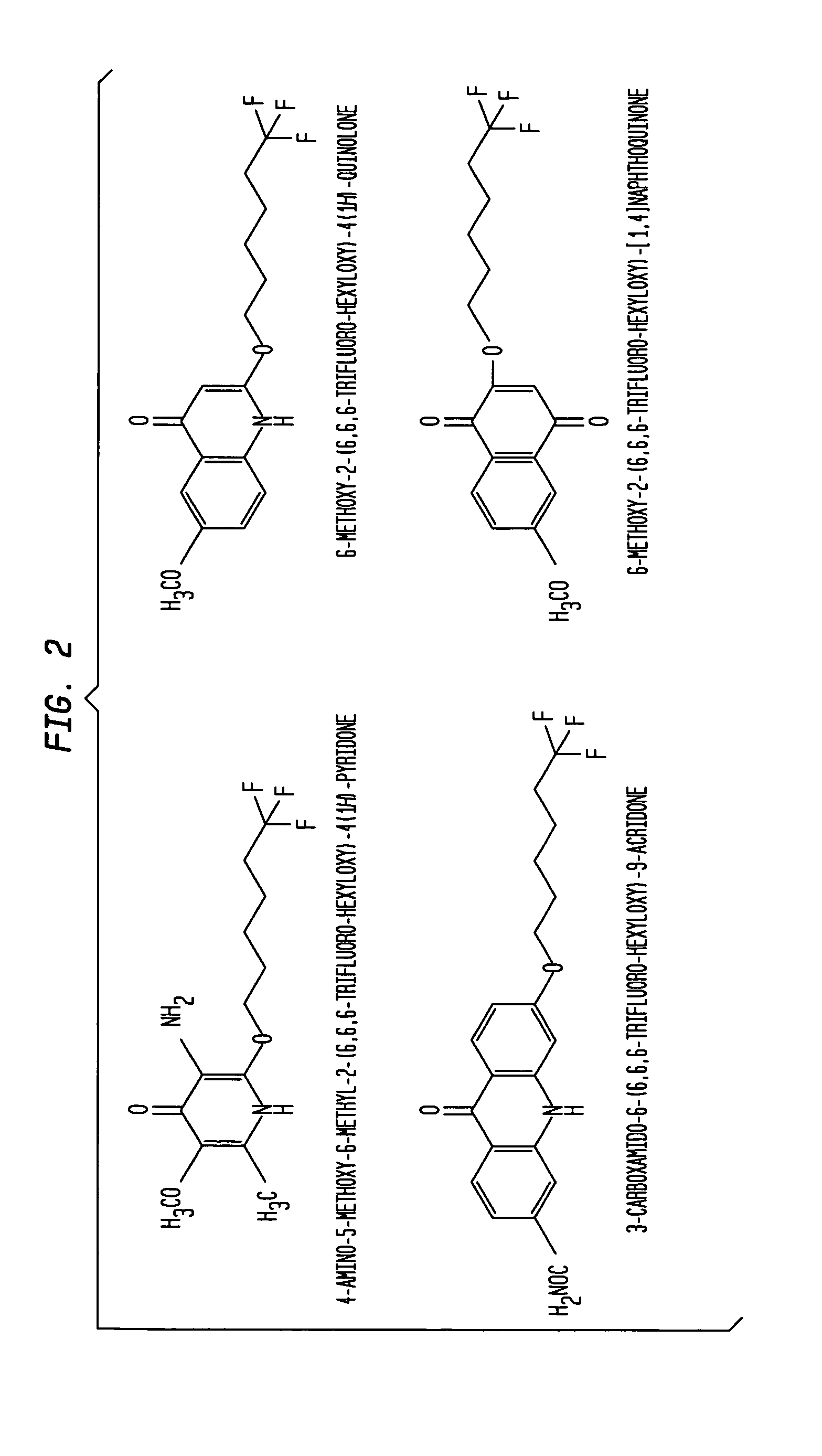
Aromatic ketones and uses thereof
InactiveUS7829578B1Improves antimalarial potencyEnhanced anti-parasiticBiocideOrganic chemistryDiseaseXanthone
Aromatic ketones having an extended fluoro-alkyl or fluoro-alkoxy moiety are disclosed. In particular aspects, the compounds comprise substituted 9-acridone, 9-xanthone, 4(1H)-quinolone, 4(1H) pyridone, 1,4-naphthoquinone, 9,10-anthraquinone derivatives. These preparations possess potent pharmacological activity for inhibiting malaria and mosquito-borne (Plasmodium) diseases. The haloalkyl / alkoxy aromatic compounds possess significant pharmacological activity, with IC50 values in the nanomolar and sub-nanomolar range, and reduced toxicity against host derived cells and tissues. Methods of using the fluoro-alkyl / alkoxy aromatic compounds in the treatment of malaria and other human and animal diseases are also disclosed. Agricultural uses of the fluoro-alkyl / alkoxy aromatic compounds, such as in control of fungal diseases and in the production of important commercial crops (apples, etc.), are also presented.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +1
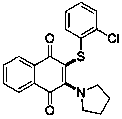
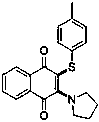
Novel Pyrrolidino-1,4-Naphthoquinone Deriviatives and their Use for Treating Malignancies and Cardiovascular Diseases
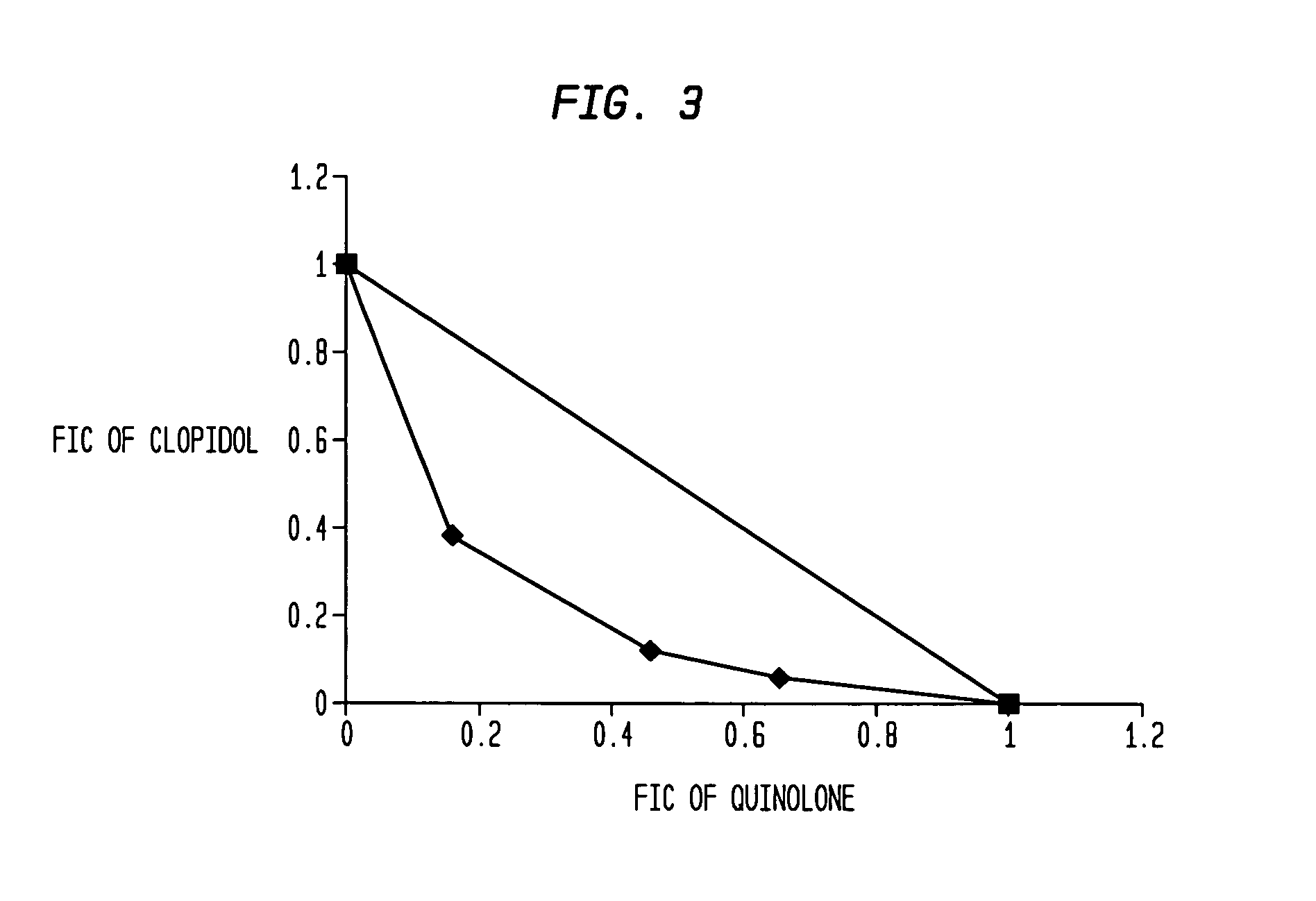
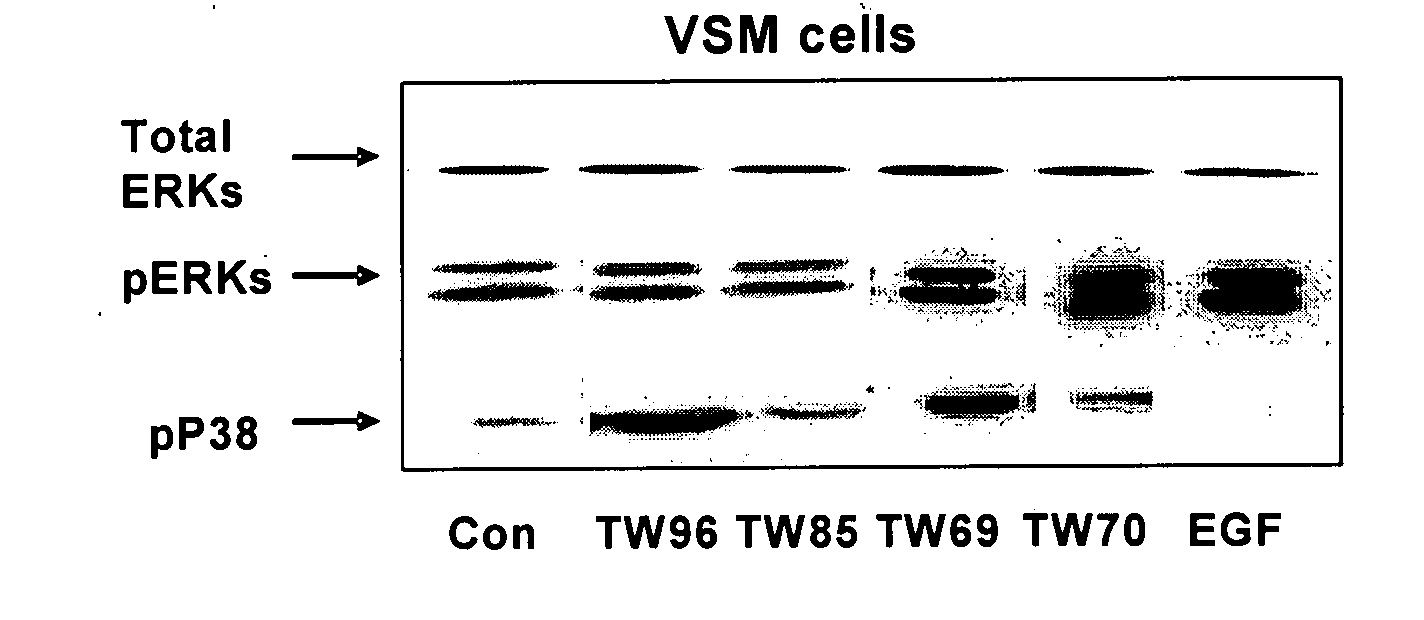
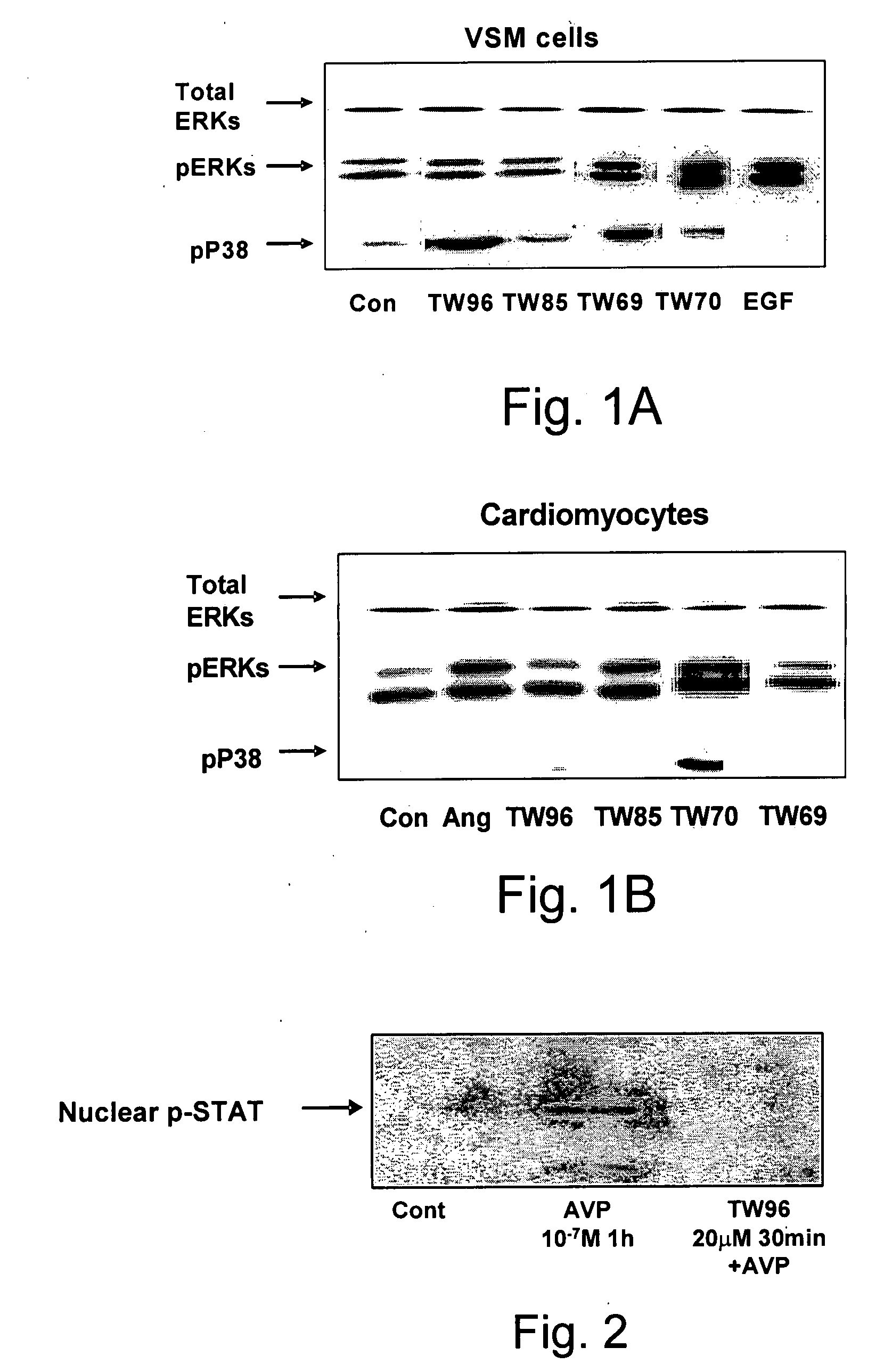
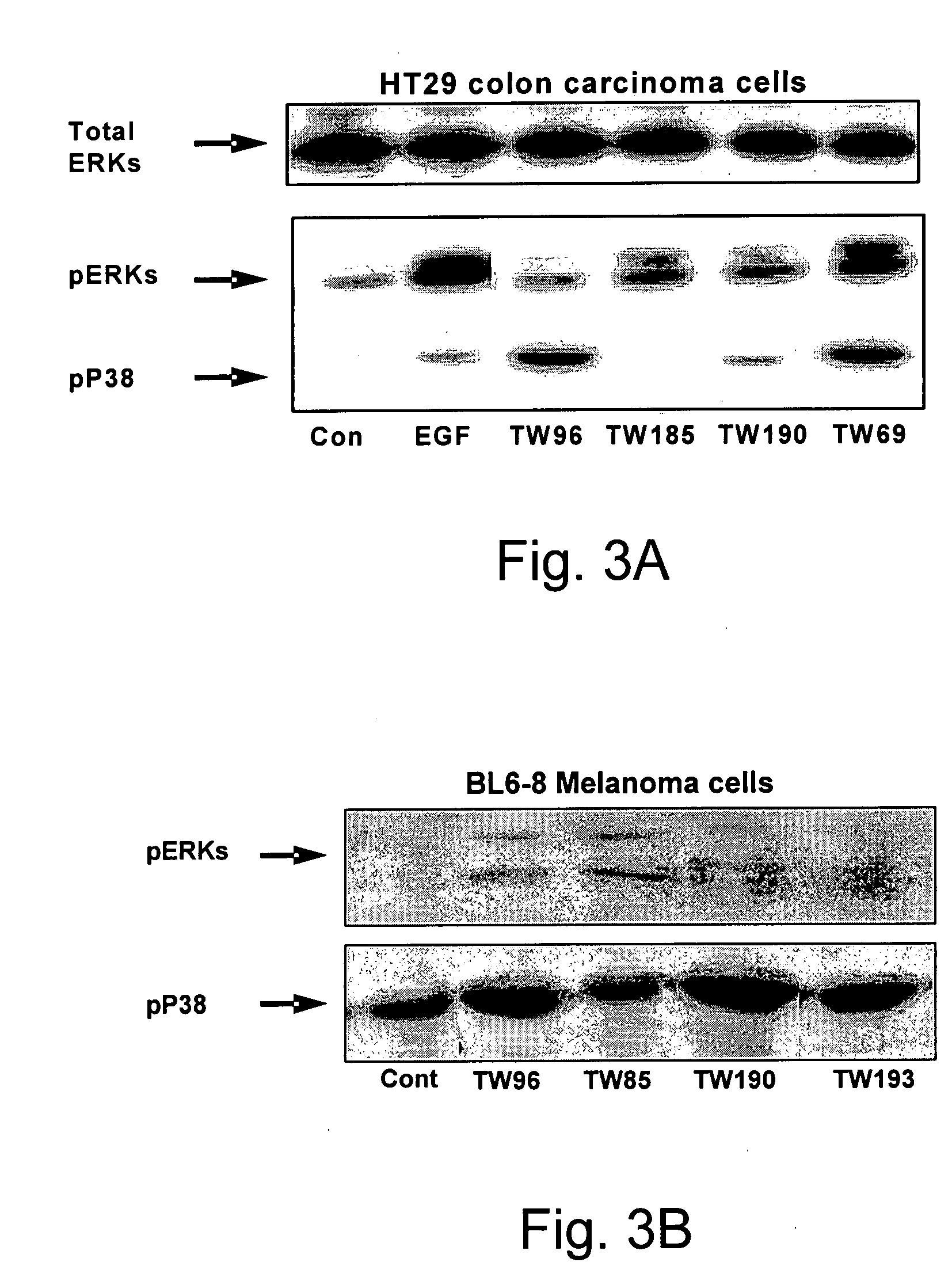
The invention provides pyrrolidino-substituted 1,4-naphthoquinone derivatives that modulate the activity of protein kinases, and it relates to the use of the derivatives in pharmaceutical compositions for treating cardiovascular disorders and malignancies. The invention particularly provides medicaments for treating disorders associated with MAPKs signaling, ERKs signaling, p38 signaling, and JNKs signaling.
Owner:BEN GURION UNIVERSITY OF THE NEGEV
Process for preparation of 2-methyl-1,4-naphthoquinone
InactiveUS20020188141A1Low costReduce usageOrganic compound preparationQuinone preparation by oxidationAcetic acidNaphthoquinone
The present invention describes a process for the preparation of 2-Methyl-1,4-naphthoquinone by oxidising 2-metbylnaphthalene with hydrogen peroxide in the presence of acetic acid.
Owner:COUNCIL OF SCI & IND RES
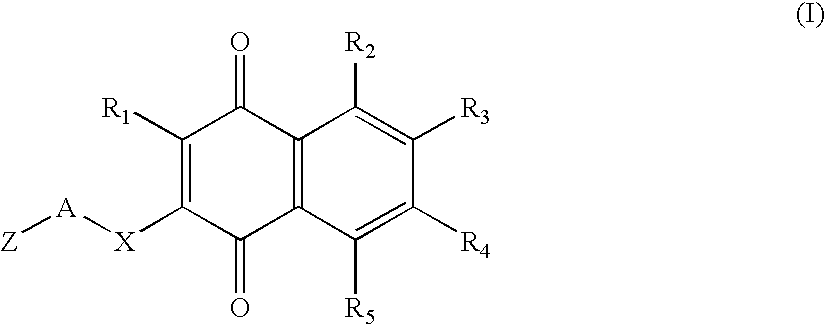
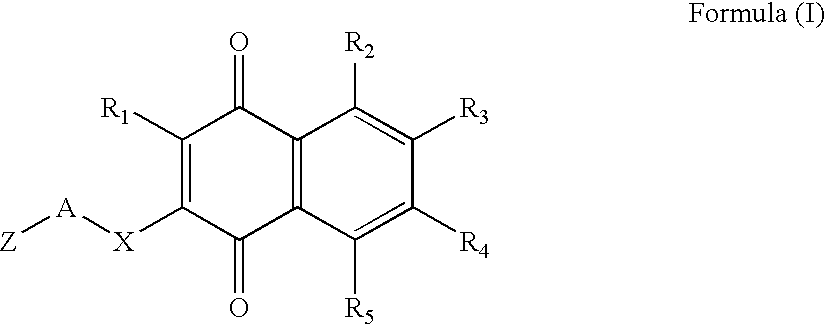
Type 1, 4-naphtoquinone compounds, compositions comprising them and use of these compounds as Anti-cancer agents
This invention relates to compounds with the formula (I) given below or one of their pharmaceutically acceptable salts, as a medicine; Formula (I) of pharmaceutical compositions comprising one or more compounds with Formula (I) as active constituent, use of compounds with Formula (I) for the preparation of compositions designed to prevent or treat at least one illness involving an abnormal cellular proliferation, pro-apoptotic compositions and / or anti-proliferative compositions comprising at least one compound with Formula (I) / and the use of compounds with formula (I) as pro-apoptotic and / or anti-proliferative agents.
Owner:FLUOFARMA +2
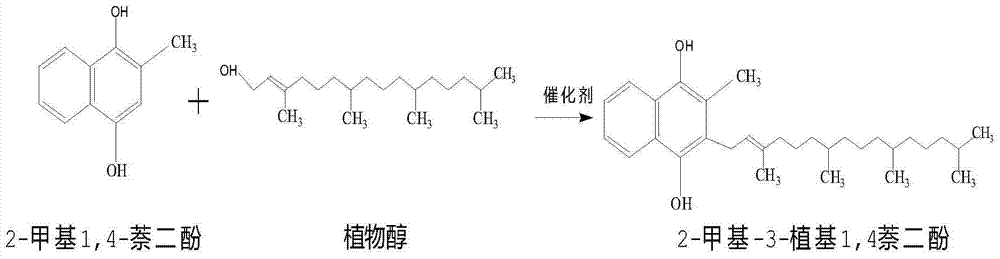
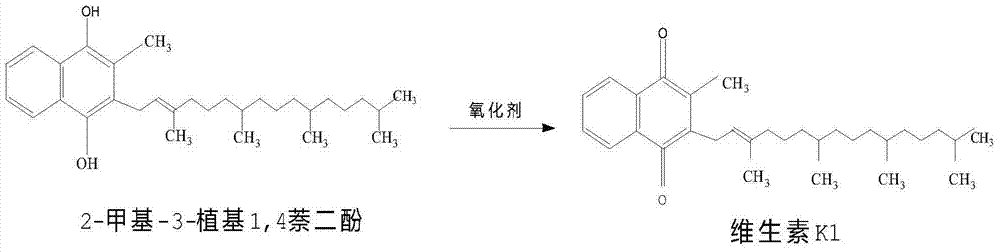
Method for synthesizing vitamin K1
InactiveCN104744230AEasy to operateMild reaction conditionsQuinone preparation by oxidationNaphthoquinoneSolvent
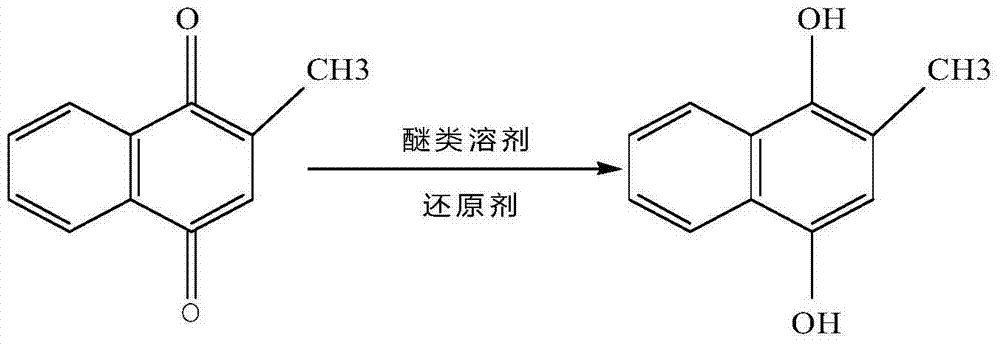
The invention discloses a method for synthesizing vitamin K1, relating to the field of synthesis of organics. The method comprises the following steps: reducing 2-methyl1,4-naphthalenediol in a certain solvent in the presence of a reducing agent under certain conditions to generate 2-methyl-1,4-hydronaphthoquinone; then, adding pohytol and reacting under certain conditions in the presence of a proper catalyst to synthesize 2-methyl3-phytyl1,4-naphthalenediol; oxidizing with a proper oxidizing agent to generate vitamin K1; and carrying out vacuum concentration to obtain finished vitamin K1. When the method is used for synthesizing vitamin K1, 2-methyl1,4-naphthoquinone is used as a raw material, no hydroxyl group protection is needed. Therefore, the method disclosed by the invention has the characteristics of simple process, mild reaction conditions, high yield, high product purity and high product yield; and in addition, the production cost is low, and therefore the method is very suitable for industrial production.
Owner:安徽万和制药有限公司
7-(4-chlorphenyl)-5,6-dihydro-7ah-benzo[h]1,2,4-triazolo[3,4-b]quinazoline-5,6-diketone and synthetic method thereof
The invention provides 7-(4-chlorphenyl)-5,6-dihydro-7aH-benzo[h]1,2,4-triazolo[3,4-b]quinazoline-5,6-diketone and a synthetic method thereof. The method comprises the following steps of: uniformly mixing 3-amino-1,2,4-triazole, p-chlorobenzaldehyde, 2-hydroxyl-1,4-naphthaquinone and p-methylbenzene sulfonic acid with a usage amount of catalyst, heating and stirring, controlling the temperature at 110-125 DEG C and reacting for 2.5-3 hours. The 7-(4-chlorphenyl)5,6-dihydro-7aH-benzo[h]1,2,4-triazolo[3,4-b]quinazoline-5,6-diketone prepared according to the invention introduces chlorine atoms, thus having stronger activity and being more beneficial to absorption. The method provided by the invention is green and environment-friendly, and has the characteristics that the raw materials are available, the operation is simple and the yield is high.
Owner:XINXIANG MEDICAL UNIV
Preparation method of thiamine 1,4-naphthoquinone compound
InactiveCN109574959AWide adaptabilityMild reaction conditionsOrganic chemistryThiamineSynthesis methods
The invention uses naphthoquinone, amine and mercaptan as raw materials, and provides a preparation method of a thiamine 1,4-naphthoquinone compound. The synthesis method has mild reaction conditions,oxygen is used as an oxidant under the condition of copper catalysis, and multiple thiamine 1,4-naphthoquinone compounds can be synthesized through a one-step reaction, and thus the method provides an efficient and practical synthesis method which is simple, convenient and efficient, cheap and easily-available in raw materials and catalysts, wide in adaptability, and has good application value and market prospect.
Owner:ZHENGZHOU UNIV
Preparation method of atovaquone
InactiveCN103570521AReduce usageReduce adverse effectsOrganic compound preparationQuinone preparation by oxidationAtovaquoneAcid catalysis
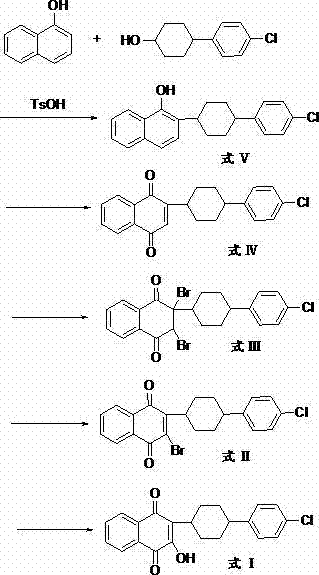
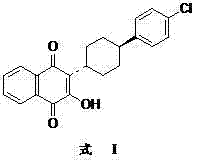
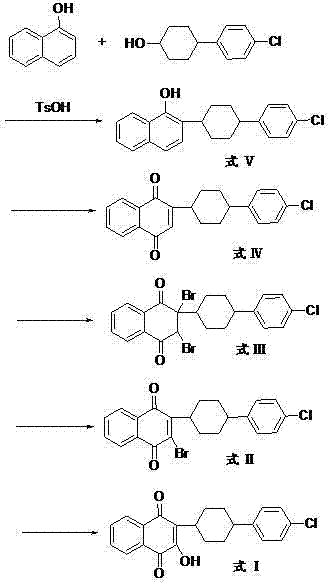
The invention discloses a preparation method of atovaquone, and belongs to the drug synthesis field. The method comprises the following steps: condensing alpha-naphthol and 4-(4-chlorophenyl)cyclohexanol under acid catalysis to obtain 2-(4-(4-chlorophenyl)cyclohexyl)-1-naphthol (formula V), oxidizing the compound represented by formula V to obtain 2-(4-(4-chlorophenyl)cyclohexyl)-1,4-naphthoquinone (formula IV), enabling the compound represented by the formula IV to react with bromine in additive reaction to obtain 2,3-dibromo-2-(4-(4-chlorophenyl)cyclohexyl)-1,4-naphthoquinone (formula III), releasing a molecule of hydrogen bromide to obtain 3-bromo-2-(4-(4-chlorophenyl)cyclohexyl)-1,4-naphthoquinone (formula II), hydrolyzing to obtain the atovaquone (formula I). Compared with the prior art, the method disclosed by the invention is simple in process, the expensive silver nitrate is prevented from using in the preparation process; and meanwhile, the yield is improved, the pollution to the environment is reduced, and the method has good popularization and application value.
Owner:SHANDONG LUKANG SHELILE PHARMA
2-(3-amino-2-oxoindolin-3-yl)-3-hydroxyl-1,4-naphthoquinone derivative and preparation method thereof
ActiveCN104030966AShort melting rangeMild reaction conditionsPolycrystalline material growthOrganic chemistrySingle crystalSolvent
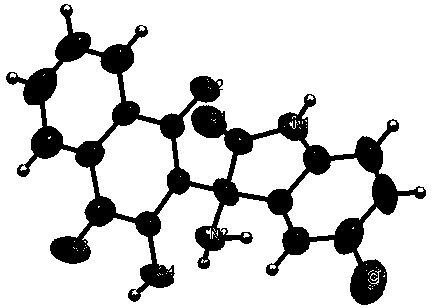
The invention provides a 2-(3-amino-2-oxoindolin-3-yl)-3-hydroxyl-1,4-naphthoquinone derivative and a preparation method thereof, and belongs to the technical field of compound synthesis. The preparation method comprises the following steps: dissolving isatin derivative as shown in the formula (I), 2-hydroxyl-1,4-naphthoquinone as shown in the formula (II) and an ammonia source as shown in the formula (III) in a solvent; sufficiently stirring to react under a reflux state; tracking by TLC (thin layer chromatography) until the raw materials are completely reacted; cooling, performing suction filtering, and baking to obtain the target product. The preparation method has the advantages of mild reacting condition, simple operation, convenience in post-treatment and high yield which is over 98 percent at most and the melting range of the prepared product is short; the structures of corresponding compounds are certified by IR (infrared radiation), 1HNMR (1H nuclear magnetic resonance), 12CNMR (12C nuclear magnetic resonance) and ESI-MS (electrospray ionization mass spectrometry), and monocrystal can be cultured by a part of target products.
Owner:乐陵市博奥泡沫制品有限公司
Method for preparing 2-methyl-1,4-naphthoquinone through microwave radiation
InactiveCN105669413AHigh activityHeating evenlyQuinone preparation by oxidationPolystyrenePeroxy acid
The invention relates to a method for preparing 2-methyl-1,4-naphthoquinone through microwave radiation. The 2-methyl-1,4-naphthoquinone is prepared from beta-methylnaphthalene in a solvent acetate acid gracial under the action of microwave radiation with a polystyrene type polymeric peroxy acid as an oxidant, and the yield reaches 65.0% or above. Microwave radiation heating is adopted, so the reaction time is obviously shortened, and the product yield is improved; and the polymeric peroxy acid oxidant is adopted, so the method has the advantages of high product yield, convenient post-treatment , avoiding of heavy metal pollution of chromium salt oxidants, environmental protection, and important application values.
Owner:LIAOCHENG UNIV
12-(2-fluorophenyl)-benzo [h][1,3] methylenedioxy [4,5-b] acridine-10,11-diketone and synthesis method thereof
InactiveCN104610271AHigh activityProfit absorptionOrganic chemistryAntineoplastic agentsAcridineKetone
The invention discloses 12-(2-fluorophenyl)-benzo [h][1,3] methylenedioxy [4,5-b] acridine-10,11-diketone and a synthesis method thereof, and belongs to the technical field of dihydroketoacridine derivatives. The method comprises the steps of uniformly mixing 3,4-methylenedioxy aniline, 2-fluorobenzaldehyde, 2-hydroxyl-1,4-naphthaquinone, a catalyst and toluene sulfonic acid, performing heating and stirring, controlling the temperature to be 115-130 DEG C, and performing a reaction for 3-5h. Fluorine atom and methylenedioxy structures are introduced into 12-(2-fluorophenyl)-benzo [h][1,3] methylenedioxy [4,5-b] acridine-10,11-diketone, so that 12-(2-fluorophenyl)-benzo [h][1,3] methylenedioxy [4,5-b] acridine-10,11-diketone has higher activity, and more facilitates absorption. The synthesis method has the characteristics that the synthesis method is simple to operate, high in yield, green and environment-friendly, and raw materials are easy to obtain.
Owner:XINXIANG UNIV
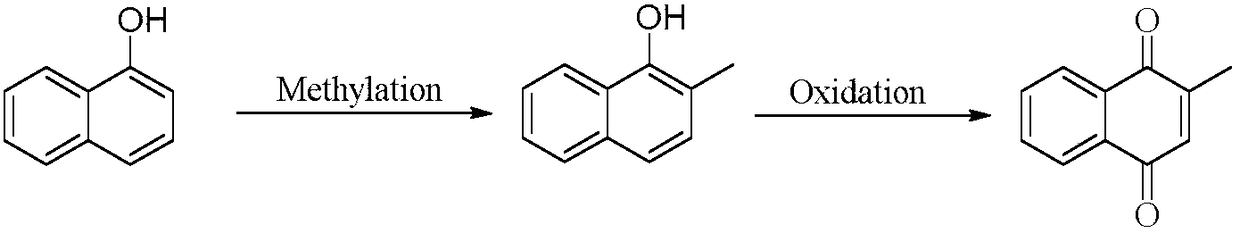
Preparation method of 2-hydroxy-1, 4-naphthoquinone
InactiveCN101759547ANo pollutionThe synthesis process is simpleQuinone preparation by oxidationOrganic-compounds/hydrides/coordination-complexes catalystsNaphthoquinonePhthalocyanine
The invention discloses a preparation method of 2-hydroxy-1, 4-naphthoquinone, which is characterized by mixing naphthol and hydroxide, taking metal phthalocyanine dissolved by hydrophobic ionic liquid as catalyst to have oxygenic reaction, and leaching and drying the reactant to prepare 2-hydroxy-1, 4-naphthoquinone. Compared with the prior art, the invention has simple synthesis process, low cost and easy taking of raw material, low reaction cost and high yield, and the catalyst can be recycled to used and can not pollute environment.
Owner:EAST CHINA NORMAL UNIV
Method for preparing vitamin K3
InactiveCN105037125AAvoid sublimationInhibitory reactivityQuinone preparation by oxidationQuinone separation/purificationVitamin K3Sodium bisulfate
The invention provides a method for preparing vitamin K3, and relates to vitamin K3. The method for preparing vitamin K3 with the chemical name of 2-methyl-1,4-naphthoquinone includes the following steps: dissolving 2-methylnaphthalene into a hydrocarbon solvent; adding sodium dodecyl sulfate and chromium ion oxidation liquid for an oxidation reaction; after completion of the reaction, purifying the reaction solution to obtain vitamin K3 with the chemical name of 2-methyl-1,4-naphthoquinone. The method for preparing vitamin K3 with the chemical name of 2-methyl-1,4-naphthoquinone sodium bisulfate includes the following steps: dissolving 2-methyl-1,4-naphthoquinone into a mixed solvent of water and ethyl alcohol; then adding sodium bisulfite for a sulfonation reaction; after completion of the reaction, purifying the reaction solution to obtain vitamin K3 with the chemical name of 2-methyl-1,4-naphthoquinone sodium bisulfate. According to the invention, the method is high in reaction selectivity, mild in reaction conditions, short in operation steps, green and environment-friendly, and stable in process; the product purity is 97% or above, and the total yield is 79% or above.
Owner:COMPREHENSIVE TECH SERVICE CENT OF QUANZHOU ENTRY EXIT INSPECTION & QUARANTINE BUREAU OF P R C
Corrosion inhibiting self-protecting coatings
ActiveUS20180141313A1Avoid corrosionEnvironment safetyLiquid surface applicatorsAnti-corrosive paintsPreservativeSalt water
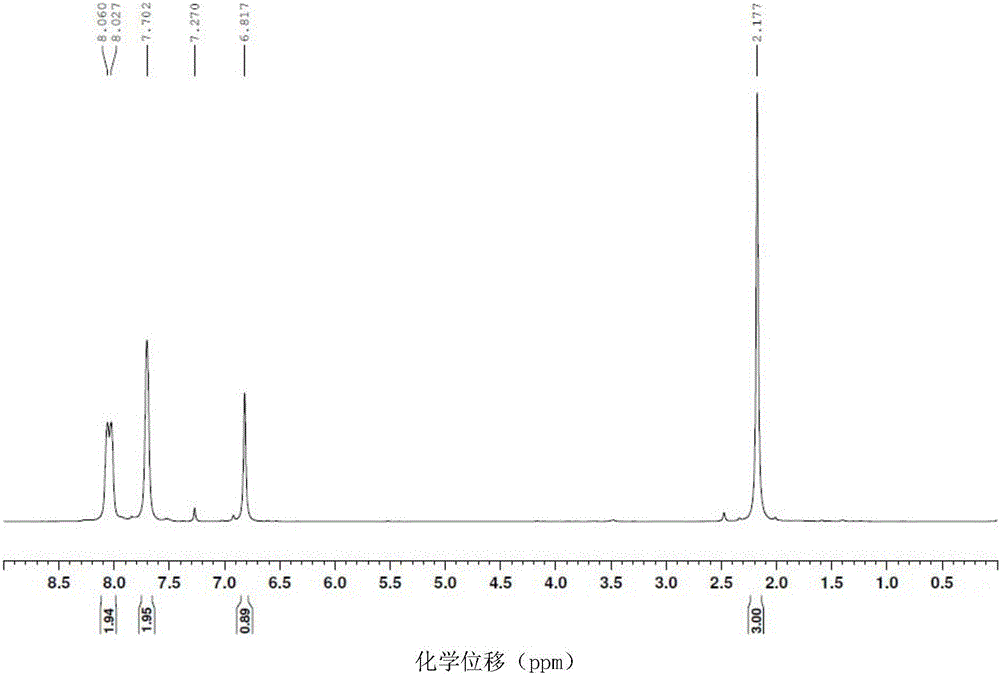
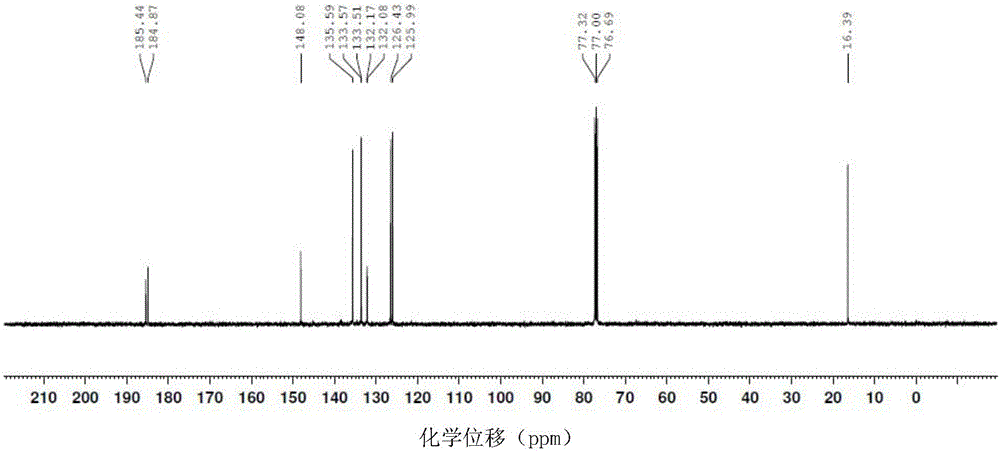
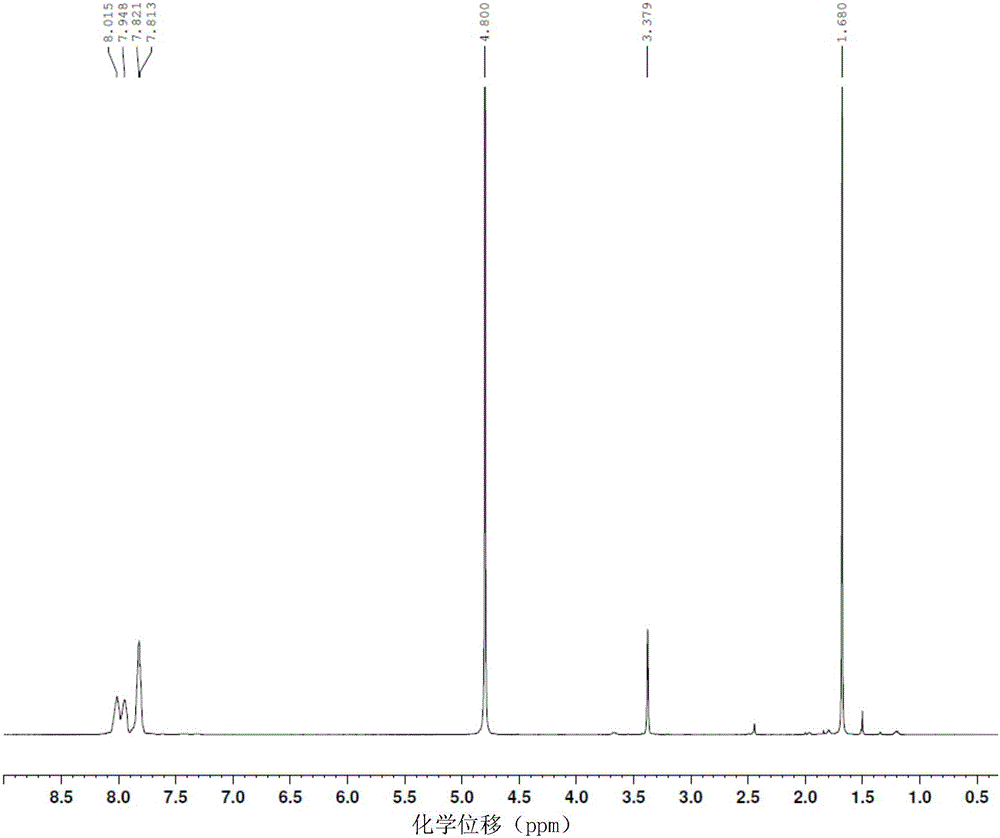
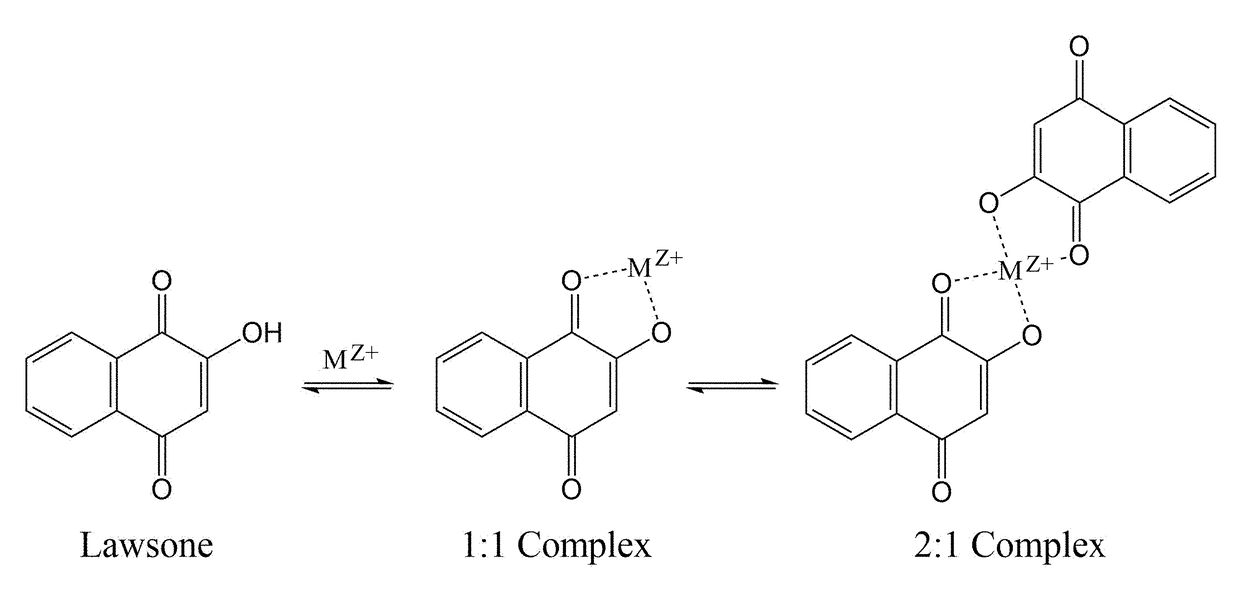
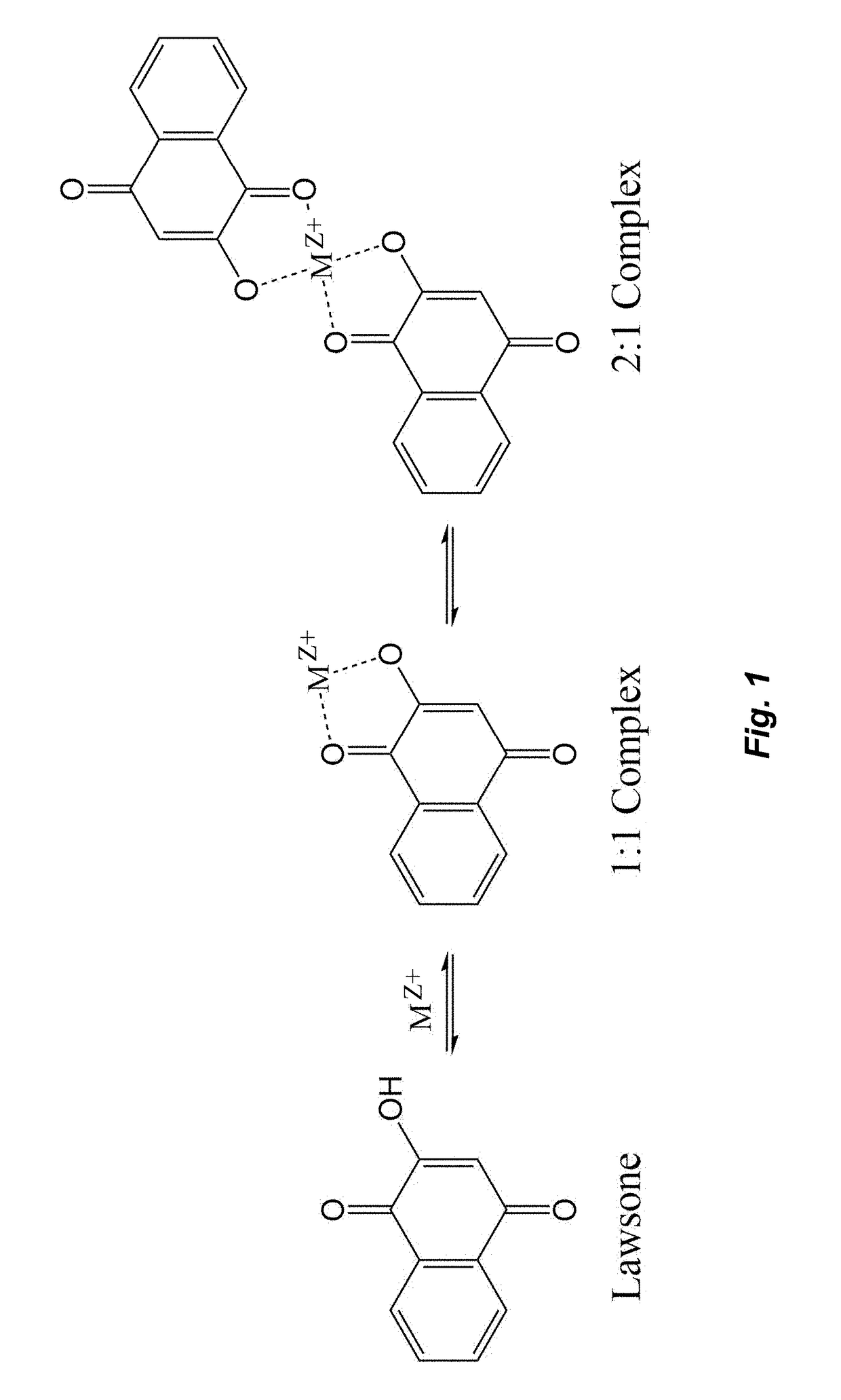
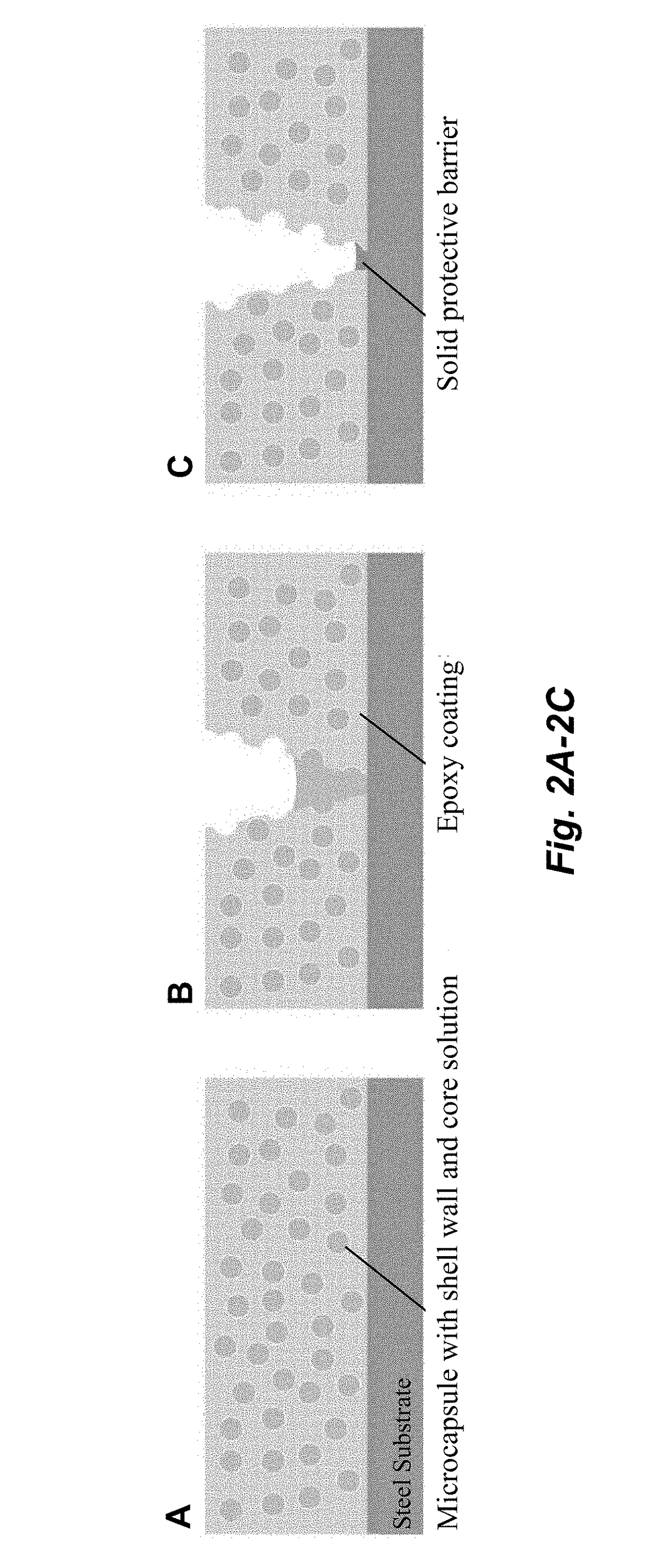
The corrosion of unprotected steel substrates causes damage that is costly to repair or replace. Current protective coatings predominately rely on environmentally harmful anticorrosive agents and toxic solvents to protect the underlying substrate. The use of lawsone (2-hydroxy-1,4-napthoquinone) together with a environmentally benign epoxy coating provides an environmentally-friendly alternative for common protective coatings. Microencapsulated lawsone embedded coatings allows the anticorrosive agent to remain dormant until released by damage and is then deposited directly onto the steel substrate. Both visual and electrochemical analysis shows that this self-protective scheme leads to 60% corrosion inhibition in a neutral salt water solution.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
2-octyl sulfoxide-1,4-naphthoquinone compound
ActiveCN105541679AHigh anticancer activityOrganic compound preparationSulfide preparationSulfurNaphthoquinone
The invention discloses a 2-octyl sulfoxide-1,4-naphthoquinone compound which solves the problem that the research on 1,4-naphthoquinone without any substituent groups at the fifth bit and the eighth bit is little. The structural formula of the compound can be seen in the description. No substituent groups are arranged at the fifth bit or the eighth bit of the compound, the second bit is substituted by sulfydryl, sulfur at the second bit is oxidized into sulfoxide, and the naphthoquinone compound can have more excellent anti-cancer activity.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Naphthoquinone derivatives useful for prevention of amyloid deposits and treatment of diseases involving amyloidogenesis
InactiveUS20140213627A1Low toxicityImprove bioavailabilityBiocideNervous disorderNaphthoquinoneSenile dementia
Owner:RAMOT AT TEL AVIV UNIV LTD
Preparation method for 2-methyl-1,4-naphthoquinone
ActiveCN109384659AHigh yieldMild reaction conditionsOrganic compound preparationQuinone preparationMethyl groupHydrolysis
The invention relates to a preparation method for 2-methyl-1,4-naphthoquinone. The preparation method utilizes phthalic acid diester and 3-cyanobutyrate to prepare 2-methyl-1,4-naphthoquinone throughcondensation, hydrolysis and decarboxylation and hydrogen cyanide removal under the action of strong alkali. The preparation method is easy in raw material obtaining, cheap in price and high in reaction atomic economy; and the 3-cyanobutyrate can be obtained through addition reaction of by-product hydrogen cyanide and 2-crotonate; and the preparation method is easy to operate, less in waste wateramount and green and environmentally friendly in technology, and therefore the discharging of waste water without heavy metals can be realized, and industrial production can be achieved.
Owner:XINFA PHARMA
Novel crystalline forms of atovaquone
The present invention relates to two novel and stable crystalline forms of atovaquone, to processes for their preparation and to pharmaceutical compositions comprising them. The present invention also provides crystalline particles of atovaquone having a specific surface area of from about 0.7 m2 / g to about 4 m2 / g, methods for the manufacture of said crystalline particles and pharmaceutical compositions comprising said crystalline particles. The present invention further provides an improved and commercially viable process for preparation of atovaquone substantially free of its undesired isomeric impurity, namely cis-2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthoquinone.
Owner:HETERO DRUGS LTD
Synthesis method of compounds of 5-benzoyl-1, 4-naphthaquinone with similar gossypol effect
InactiveCN102584560AReasonable choiceStable physical and chemical propertiesQuinone preparation by oxidationSynthesis methodsKetone
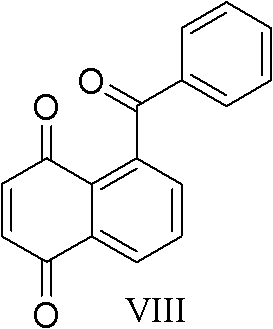
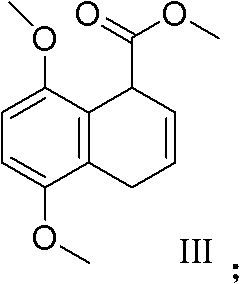
The invention discloses a practical synthesis method of compounds of 5-benzoyl-1, 4-naphthaquinone with a similar gossypol effect. The practical synthesis method comprises the steps that: a) malonic acid and acrolein take condensation reaction to obtain a compound of pentadienoic acid shown in the formula I; b) the compound shown in the formula I takes reaction with p-benzoquinone to obtain a compound of cis-5, 8-diketone-1,4,6,7,9,10-hexahydronaphthalene-beta-carboxylic acid shown in the formula II; c) the compound shown in the formula II is subjected to methylation to obtain a compound of 5,8-dimethoxy-1,4-dihydronaphthalene-1-carboxylic acid-methyl ester shown in the formula III; d) the compound shown in the formula III takes dehydro-aromatization reaction to obtain a compound of 5,8-dimethoxy naphthoate shown in the formula IV; e) the compound shown in the formula IV is hydrolyzed to obtain a compound of 5,8-dimethoxy naphthoyl naphthoic acid shown in the formula V; f) the compound shown in the formula V is subjected to chlorination to obtain a compound of 5,8-dimethoxy naphthoyl chloride shown in the formula VI; g) the compound shown in the formula VI and benzene are subjected to Friedel-Crafts acidylation to obtain a compound of 5-benzoyl-1,4-dimethoxy naphthalene shown in the formula VII; and h) the compound shown in the formula VII is oxidized to obtain a compound of 5-benzoyl-1,4-naphthoquinone shown in the formula VIII.
Owner:SOUTHEAST UNIV
Process for preparation of atovaquone and novel intermediates thereof
Disclosed herein is a novel process for preparation of atovaquone. The process includes reacting 1,4-naphthoquinone with trans-4-(4-chlorophenyl) cyclohexane carboxylic acid followed by halogenation to obtain a dihalo-compound. Further, dehydrohalogenation of the dihalo-compound produces a monohalogeno-compound which under goes hydrolysis to produce atovaquone. The invention also discloses atovaquone in a substantially pure and well defined polymorphic form designated as “Form IPCA-ATO,” and the preparation thereof.
Owner:IPCA LAB LTD
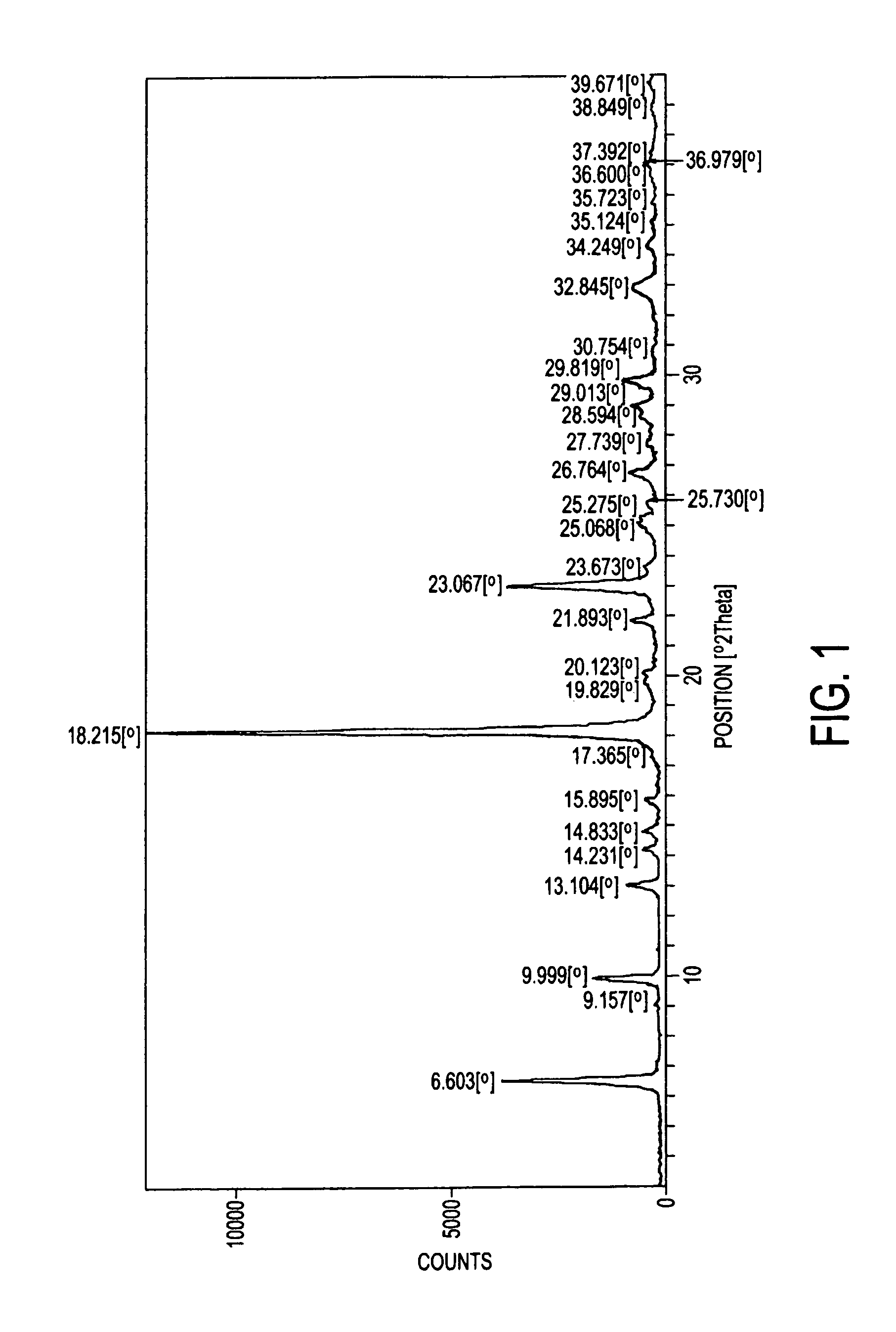
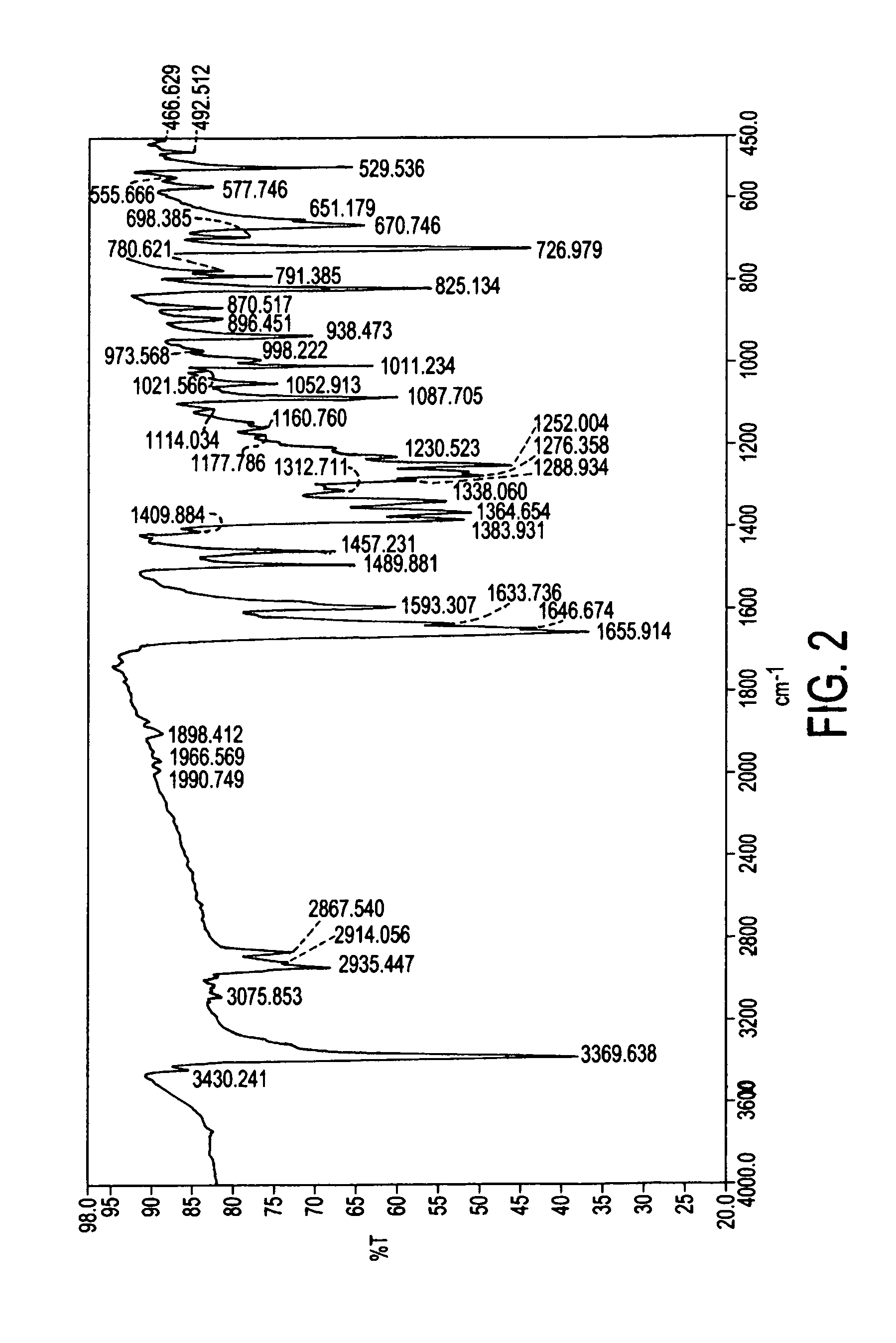
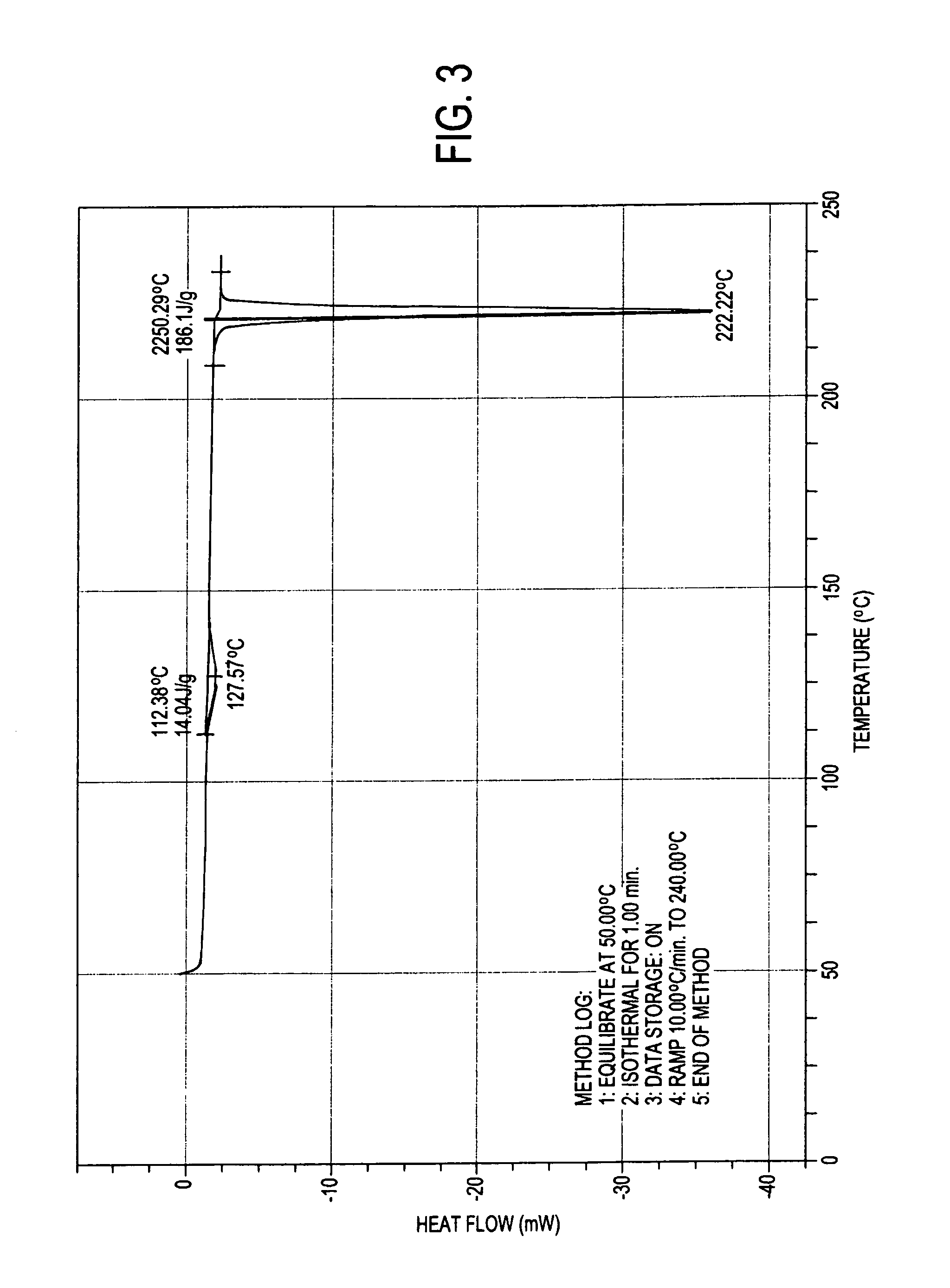
Preparation method of 2-acetoxyl-1,4-naphthoquinone
InactiveCN101844982ASimple processGood repeatabilityOrganic compound preparationCarboxylic acid esters preparationWater bathsAcetic anhydride
The invention relates to a preparation method of 2-acetoxyl-1,4-naphthoquinone, comprising the following steps of: (A) adding 2- hydroxyl-1,4-naphthoquinone into acetic anhydride; (B) adding concentrated sulfuric acid under the condition of water bath heating; (C) cooling a reactant at normal temperature; (D) adding a reacting solution into cold water and carrying out suction filtering to obtain a 2-acetoxyl-1,4-naphthoquinone crude product; and (E) re-crystalizing by using an organic solvent. The method is simple and feasible; and the obtained product used as a pesticide has broad application prospect.
Owner:TIANJIN POLYTECHNIC UNIV
Process for Industrial Production of 2-Methyl-1,4-Naphthaquinone
InactiveUS20130324747A1Low yieldReduce yieldQuinone preparation by oxidationAcetic acidNaphthoquinone
Owner:TUBITAK
Anti-allergic agent
ActiveUS20090281187A1Potent degranulation-inhibiting effectBiocideKetone active ingredientsVitamin K3BULK ACTIVE INGREDIENT
The inventors have found that vitamin K3 and vitamin K5 which may be used in pharmaceuticals and foods or ACNQ, DHNA, or the like which can stimulate the growth of bifidobacteria can inhibit degranulation of basophil-like cells, exhibit a potent degranulation-inhibiting effect, and are useful anti-allergic agents or foods. The present invention provides an anti-allergic agent containing, as an active ingredient, one or more species selected from among 2-amino-3-carboxy-1,4-naphthoquinone, 1,4-dihydroxy-2-naphthoic acid, 1,4-naphthoquinone, 4-amino-2-methyl-1-naphthol, 2-methyl-1,4-naphthoquinone, 2-amino-3-chloro-1,4-naphthoquinone, and a salt thereof.
Owner:MEIJI CO LTD
Catalyst for preparing 1,4-naphthoquinone through liquid-phase oxidation of naphthalene as well as preparation method and application of catalyst
ActiveCN109876849AImprove conversion rateHigh selectivityMolecular sieve catalystsQuinone preparation by oxidationStrong acidsPollution
The invention discloses a catalyst. The catalyst comprises a carrier and an active component, wherein the carrier is a molecular sieve; the active component is a transition metal oxide modified by strong acid; the mass percentage content of the active component is 0.5%-50% based on the mass of the catalyst. The catalyst is used for preparing 1,4-naphthoquinone by liquid-phase oxidation of naphthalene. The invention also discloses a preparation method of the catalyst and application of the catalyst to preparation of 1,4-naphthoquinone by liquid-phase oxidation of naphthalene, the conversion rate of naphthalene and the selectivity of 1,4-naphthoquinone are effectively improved, the reaction conditions are mild, the operation is simple, and the catalyst is easy to prepare, pollution-free andrecyclable.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
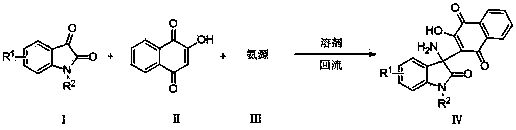
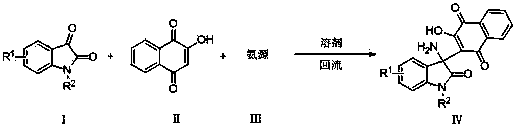
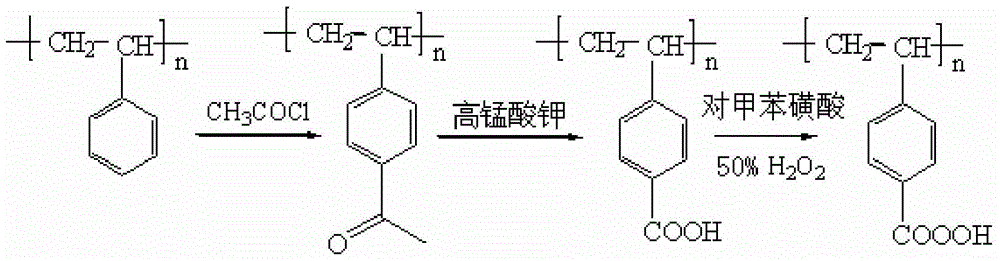
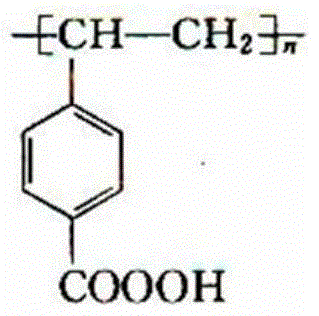
![12-(2-fluorophenyl)-benzo [h][1,3] methylenedioxy [4,5-b] acridine-10,11-diketone and synthesis method thereof 12-(2-fluorophenyl)-benzo [h][1,3] methylenedioxy [4,5-b] acridine-10,11-diketone and synthesis method thereof](https://images-eureka.patsnap.com/patent_img/b61ecf96-9fcc-4ae3-ae15-bd62743e13ee/DEST_PATH_IMAGE001.PNG)
![12-(2-fluorophenyl)-benzo [h][1,3] methylenedioxy [4,5-b] acridine-10,11-diketone and synthesis method thereof 12-(2-fluorophenyl)-benzo [h][1,3] methylenedioxy [4,5-b] acridine-10,11-diketone and synthesis method thereof](https://images-eureka.patsnap.com/patent_img/b61ecf96-9fcc-4ae3-ae15-bd62743e13ee/DEST_PATH_IMAGE003.PNG)