Method for preparing vitamin K3
A vitamin and methyl technology, applied in the field of vitamin K3 preparation, can solve the problems of poor reaction selectivity, complicated post-treatment, high reaction temperature, etc., and achieve the effects of mild reaction conditions, stable process, and simplified operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0030] The preparation of embodiment 12-methyl-1,4-naphthoquinone
[0031] At 15°C, after dissolving 2-methylnaphthalene (1kg) in n-hexane (2L), add sodium dodecylsulfonate (0.1Kg) and 2.75mol / L chromium ion oxidation solution (35L), Stir mechanically, after reacting for 3 hours, put the reaction solution in the refrigerator to freeze (-18°C) for crystallization for 6 hours, filter with suction, wash with water to obtain a filter cake, which is confirmed to be 2-methyl-1,4-naphthalene by NMR detection The quinone was weighed after drying, the yield was 84.5%, and the purity was 97% when detected by high performance liquid chromatography.
Embodiment 1
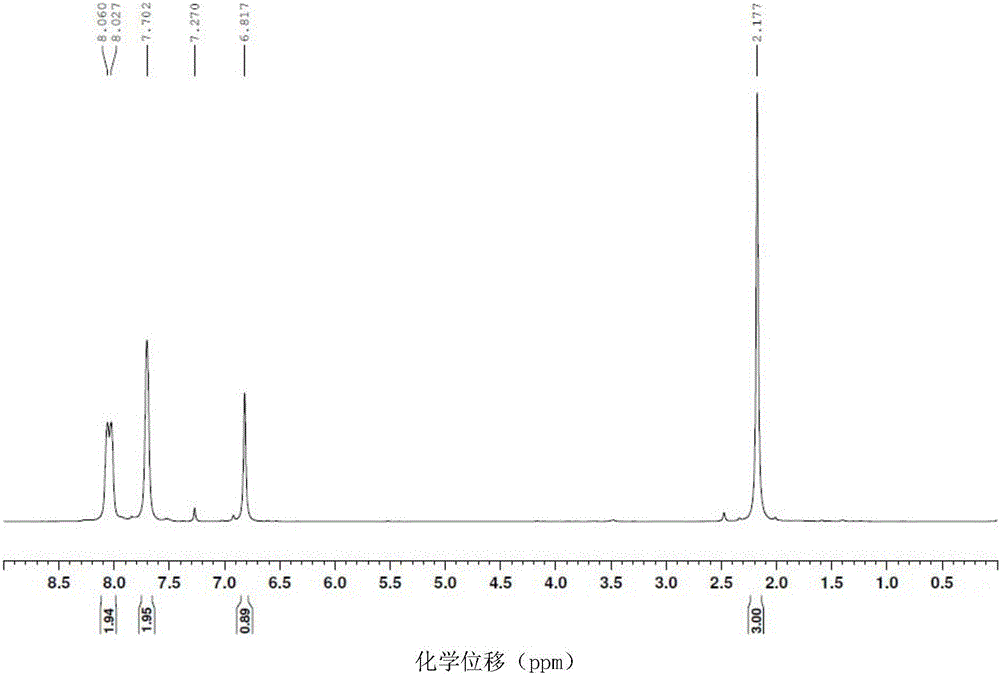
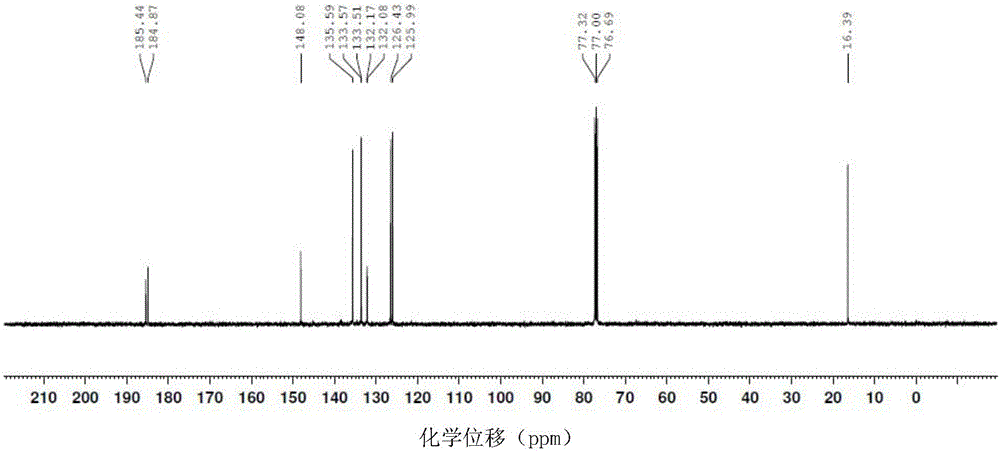
[0032] The proton nuclear magnetic resonance spectra of the 2-methyl-1,4-naphthoquinone prepared in Example 1 can be found in figure 1 , the carbon nuclear magnetic resonance spectrogram of the 2-methyl-1,4-naphthoquinone that embodiment 1 prepares sees figure 2 .
Embodiment 22
[0033] The preparation of embodiment 22-methyl-1,4-naphthoquinone
[0034] At 45°C, after dissolving 2-methylnaphthalene (1kg) in n-hexane (2L), add sodium dodecylsulfonate (0.1kg) and 2.75mol / L chromium ion oxidation solution (14L), Stir mechanically, react for 3 hours, put the reaction solution into the refrigerator to freeze (-18°C) for crystallization for 6 hours, filter with suction, wash with water to obtain a filter cake, which is confirmed to be 2-methyl-1,4-naphthalene by nuclear magnetic resonance The quinone was weighed after drying, the yield was 89.1%, and the purity was 98.5% by high-performance liquid chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com