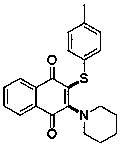
Preparation method of thiamine 1,4-naphthoquinone compound
A technology of a compound, naphthoquinone, applied in the field of preparation of thiamined 1,4-naphthoquinone compound, can solve problems such as inability to realize one-step reaction, poor reaction adaptability, etc., and achieve mild, simple and economical reaction conditions good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation method of thiamined 1,4-naphthoquinone compound, the steps are as follows:
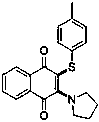
[0025] In a 25 mL reaction tube, cuprous iodide (20 mol%), 0.5 mmol of 1,4-naphthoquinone, 2 mL of solvent DMF, 0.5 mmol of p-methylbenzenethiol, and 1.75 mmol of tetrahydropyrrole were added, and the mixture was stirred in an oxygen atmosphere. , the reaction temperature was controlled to be 100 °C, and after 10 hours of reaction, silica gel column chromatography was used to separate the final product, and the yield of the final product was 80% based on the molar weight of 1,4-naphthoquinone as 100%.
[0026] The specific results are as follows:
[0027]
[0028] 1 H NMR (400 MHz, Chloroform- d ) δ 8.10 (dd, J = 7.7, 1.3 Hz, 1H), 7.93(dd, J = 7.7, 1.3 Hz, 1H), 7.69 (td, J = 7.5, 1.4 Hz, 1H), 7.60 (td, J = 7.5,1.3 Hz, 1H), 7.05 (q, J = 8.3 Hz, 5H), 3.94 – 3.85 (m, 4H), 2.28 (s, 3H), 1.85 – 1.77 (m, 4H). 13 C NMR (101 MHz, Chloroform- d ) δ 184.38, 180.28, 155.6...
Embodiment 2
[0030] The preparation method of thiamined 1,4-naphthoquinone compound, the steps are as follows:
[0031] In a 25 mL reaction tube, cuprous iodide (20 mol%), 0.5 mmol of 1,4-naphthoquinone, 2 mL of solvent DMF, 0.5 mmol of p-ethylbenzenethiol, 1.75 mmol of tetrahydropyrrole were added, and the mixture was stirred in an oxygen atmosphere. The reaction temperature was controlled to be 100 °C, and after 10 hours of reaction, the final product was obtained by separation by silica gel column chromatography, and the yield of the final product was 65% based on the molar amount of 1,4-naphthoquinone being 100%.
[0032] The specific results are as follows:
[0033]
[0034] 1 H NMR (400 MHz, Chloroform- d ) δ 8.11 (dd, J = 7.7, 1.3 Hz, 1H), 7.94(dd, J = 7.6, 1.3 Hz, 1H), 7.70 (td, J = 7.5, 1.4 Hz, 1H), 7.61 (td, J = 7.5,1.3 Hz, 1H), 7.13 – 7.04 (m, 4H), 3.97 – 3.85 (m, 4H), 2.58 (q, J = 7.6 Hz, 2H), 1.87 – 1.75 (m, 4H), 1.20 (t, J = 7.6 Hz, 3H). 13 C NMR (101 MHz, ...
Embodiment 3
[0036] The preparation method of thiamined 1,4-naphthoquinone compound, the steps are as follows:
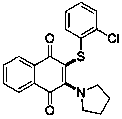
[0037] In a 25 mL reaction tube, add cuprous iodide (20 mol%), 0.5 mmol of 1,4-naphthoquinone, 2 mL of solvent DMF, 0.5 mmol of 2-chlorobenzenethiol, 1.75 mmol of tetrahydropyrrole, and stir in an oxygen atmosphere. , the reaction temperature was controlled to be 100°C, and after 10 hours of reaction, the final product was obtained by silica gel column chromatography separation, and the yield of the final product was 65% based on the molar amount of 1,4-naphthoquinone being 100%.
[0038] The specific results are as follows:
[0039]
[0040] 1 H NMR (400 MHz, Chloroform- d ) δ 8.09 (dd, J = 7.8, 1.2 Hz, 1H), 7.93(dd, J = 7.6, 1.3 Hz, 1H), 7.69 (td, J = 7.6, 1.4 Hz, 1H), 7.61 (td, J = 7.6,1.4 Hz, 1H), 7.30 (dd, J = 7.8, 1.4 Hz, 1H), 7.09 (td, J = 7.6, 1.5 Hz, 1H), 7.01 (td, J = 7.6, 1.7 Hz, 1H), 6.93 (dd, J = 7.8, 1.6 Hz, 1H), 3.96 – 3.87(m, 4H), 1.90 – 1.79...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com