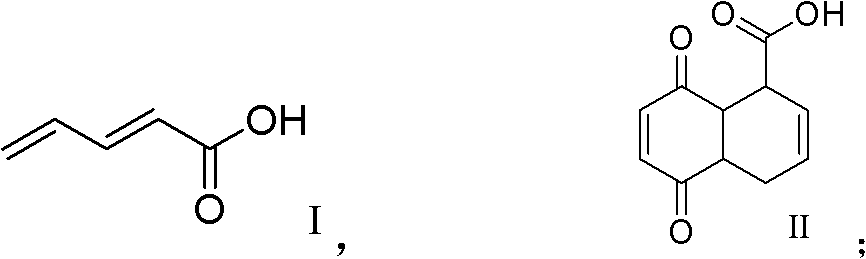Synthesis method of compounds of 5-benzoyl-1, 4-naphthaquinone with similar gossypol effect
A benzoyl and synthetic method technology, applied in the field of 5-benzoyl-1, can solve the problems of troublesome post-processing, a large amount of waste water, unfavorable preparation, etc., and achieve stable physical and chemical properties, mild reaction conditions, and reasonable reaction route selection Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
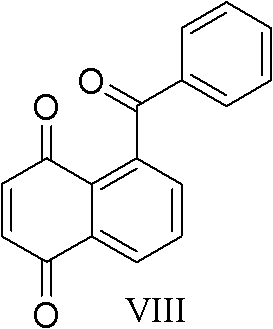
[0039] The preparation method of the formula (VIII) compound compound 5-benzoyl-1,4-naphthoquinone with gossypol-like effect of the present invention comprises the following steps:
[0040]
[0041] (a), malonic acid and acrolein obtain formula (I) compound pentadienoic acid through condensation reaction;
[0042] (b), the ring-closing reaction between the compound of formula (I) and p-benzoquinone to obtain the compound of formula (II) cis 5,8-diketone-1,4,6,7,9,10-hexahydronaphthalene-β-carboxyl acid,
[0043]
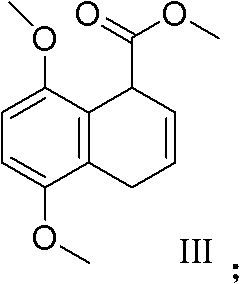
[0044] (c), formula (II) compound obtains formula (III) compound 5,8-dimethoxy-1,4-dihydronaphthalene-1-carboxylic acid methyl ester after methylation,
[0045]
[0046] (d), formula (III) compound obtains formula (IV) compound 5,8-dimethoxy naphthoic acid methyl ester through dehydroaromatization reaction,
[0047]
[0048] (e), formula (IV) compound hydrolysis obtains formula (V) compound 5,8-dimethoxy naphthoic acid,
[0049]
[0050] (f), formul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com