Method for synthesizing vitamin K1
A vitamin and methyl technology, applied in the field of synthetic vitamin K1, can solve the problems of harsh reaction conditions, low yield, simple experimental conditions, etc., and achieve the effects of mild reaction conditions, reduced operating hours, and high reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
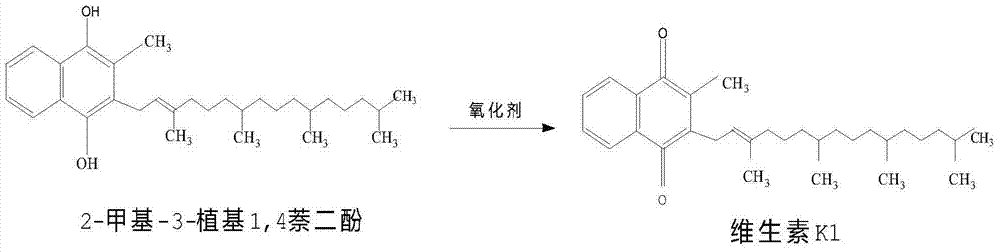
[0024] Synthesize vitamin K1 as follows:
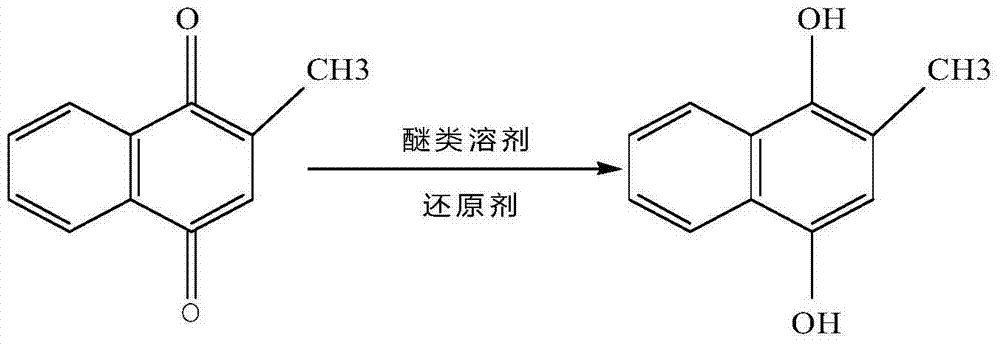
[0025] (1) Synthesis of 2-methyl 1,4-naphthodiol: Add 120g of anhydrous ether to a 500ml round-bottomed flask, then add 20g of 2-methyl-1,4-naphthoquinone, stir and dissolve, then add 80g of petroleum ether, Raise the temperature to reflux, then slowly add 200g of 25% sodium dithionite solution dropwise, and the dropwise addition is completed in about 1 hour. After the dropwise addition, keep the temperature and reflux for 3 hours; The lower aqueous layer was removed, washed twice with saturated brine, dehydrated by adding 10 g of anhydrous sodium sulfate, and filtered, and the filtrate was 2-methyl-1,4-naphthalenediol solution.
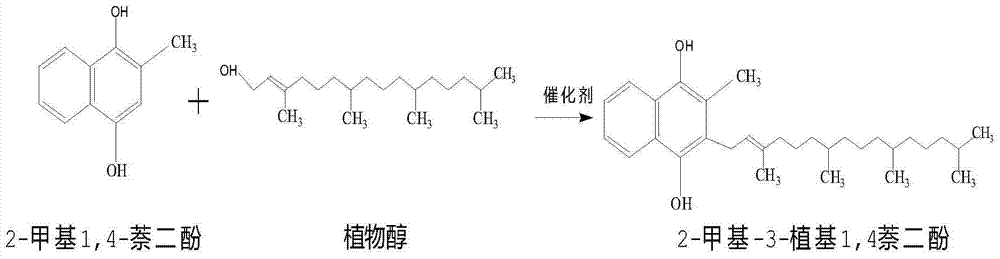
[0026] (2) Synthesis of vitamin K1: under nitrogen protection, add 2g samarium trifluoromethanesulfonate and 2g acetic acid to the above solution, stir and heat up to reflux, slowly add 60g50% phytoalcohol petroleum ether solution dropwise, after the dropwise addition is completed , keep warm and reflux for...
Embodiment 2
[0028] Synthesize sucralose-6-ethyl ester as follows:
[0029] (1) Synthesis of 2-methyl 1,4-naphthodiol: Add 150g of anhydrous ether to a 500ml round bottom flask, then add 22g of 2-methyl-1,4-naphthoquinone, stir and dissolve, then add 90g of petroleum ether, Raise the temperature to reflux, then slowly add 230g of 25% sodium dithionite solution dropwise, and the dropwise addition is completed in about 1 hour. After the dropwise addition, keep warm and reflux for 3.5 hours; The lower aqueous layer was removed, washed twice with saturated brine, dehydrated by adding 8 g of anhydrous sodium sulfate, and filtered, and the filtrate was 2-methyl-1,4-naphthalenediol solution.
[0030] (2) Synthesis of vitamin K1: under the protection of nitrogen, add 2.2g samarium trifluoromethanesulfonate and 2.5g acetic acid to the above solution, stir and heat up to reflux, slowly add 60g50% phytoalcohol petroleum ether solution dropwise, dropwise After the completion, heat preservation and re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com