Method for preparing 2-methyl-1,4-naphthoquinone through microwave radiation
A technology of microwave radiation and methylnaphthalene, applied in the preparation of quinone oxide, organic chemistry, etc., can solve the problems of producing a large amount of chromium-containing wastewater, high environmental corrosion resistance requirements, and high preparation costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
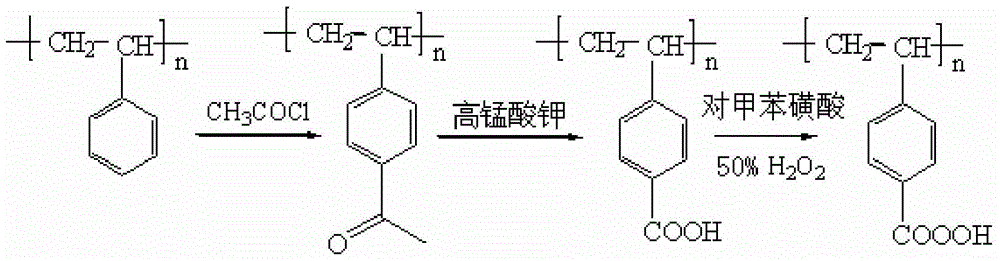
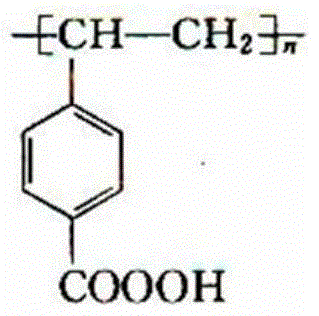
[0036] Preparation of polystyrene polymer peroxyacid:
[0037]1) Add 100g of dichloromethane and 65g of cross-linked polystyrene into a 1000mL four-necked reaction flask equipped with a reflux condenser, thermometer, and stirrer, stir and swell for 6 hours at room temperature; add 125g of anhydrous aluminum trichloride, dropwise 150g of acetyl chloride, heated up to 50°C, and reacted for 2 hours; after the reaction, washed with tetrahydrofuran, 5% hydrochloric acid, and distilled water in sequence until there were no chloride ions, washed with methanol three times, and dried in vacuum to obtain acetylated cross-linked polystyrene;
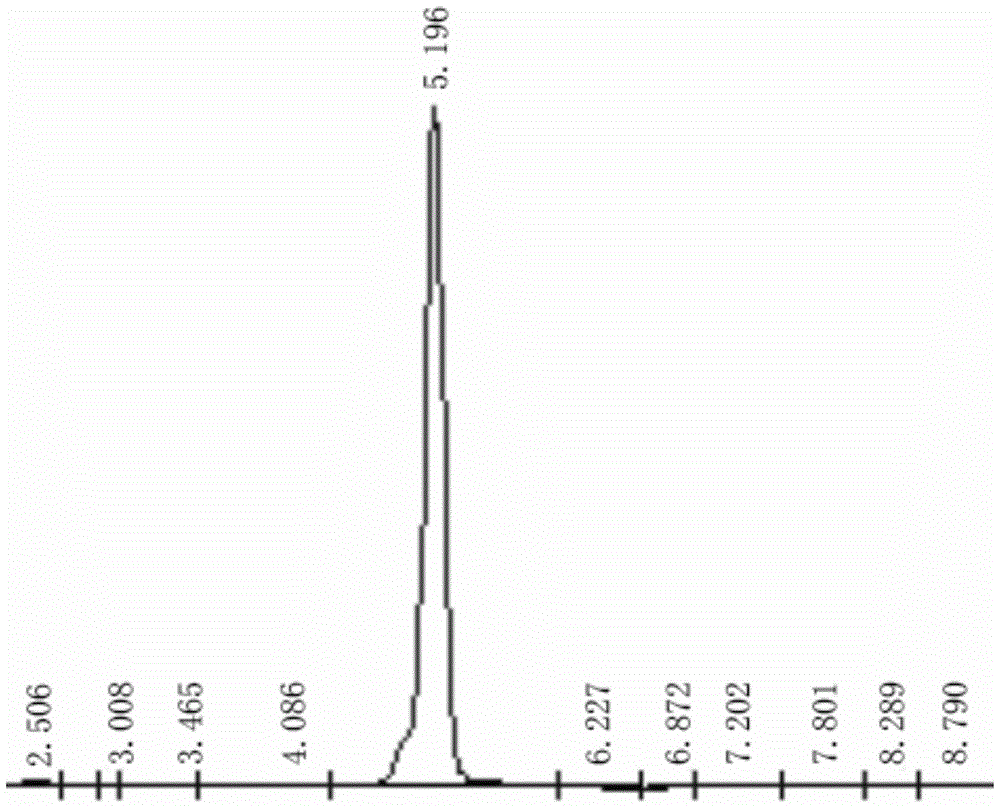
[0038] 2) Add 200mL of chlorobenzene, 400mL of water, 92g of sulfuric acid with a mass concentration of 50% in a 2000mL four-necked reaction flask equipped with a reflux condenser, a thermometer, and an agitator, and 80g of acetylated cross-linked polystyrene. After heating to boiling, Add 600g of potassium permanganate in batches, reflux for 5 hou...
Embodiment 2
[0043] Preparation of polystyrene polymer peroxyacid:
[0044] 1) Add 100g of dichloromethane and 70g of cross-linked polystyrene into a 1000mL four-necked reaction flask equipped with a reflux condenser, thermometer, and stirrer, stir and swell at room temperature for 6 hours; add 123g of anhydrous aluminum trichloride, dropwise 130g of acetyl chloride, heated up to 50°C, and reacted for 2 hours; after the reaction, washed with tetrahydrofuran, 5% hydrochloric acid, and distilled water in sequence until there was no chloride ion, washed with methanol three times, and dried in vacuum to obtain acetylated cross-linked polystyrene;
[0045] 2) Add 200mL of chlorobenzene, 400mL of water, 96g of sulfuric acid with a mass concentration of 50% in a 2000mL four-necked reaction flask equipped with a reflux condenser, a thermometer, and an agitator, and 90g of acetylated cross-linked polystyrene, and heat to boiling. Add 600g of potassium permanganate in batches, reflux for 5 hours; fi...
Embodiment 3
[0050] Preparation of polystyrene polymer peroxyacid:
[0051] 1) Add 100g of dichloromethane and 65g of cross-linked polystyrene into a 1000mL four-necked reaction flask equipped with a reflux condenser, thermometer, and stirrer, stir and swell for 6 hours at room temperature; add 125g of anhydrous aluminum trichloride, dropwise 150g of acetyl chloride, heated up to 50°C, and reacted for 2 hours; after the reaction, washed with tetrahydrofuran, 5% hydrochloric acid, and distilled water in sequence until there were no chloride ions, washed with methanol three times, and dried in vacuum to obtain acetylated cross-linked polystyrene;
[0052] 2) Add 200mL of chlorobenzene, 400mL of water, 98g of sulfuric acid with a mass concentration of 50% into a 2000mL four-necked reaction flask equipped with a reflux condenser, a thermometer, and an agitator, and 80g of acetylated cross-linked polystyrene. After heating to boiling, Add 600g of potassium permanganate in batches, reflux for 5 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com