Drug for treating allergic purpura
A kind of allergic purpura, the following technology, applied in the direction of allergic diseases, drug combination, pharmaceutical formula, etc., can solve the problems of poor curative effect and side effects of patients, and achieve the effect of good use and definite curative effect
Inactive Publication Date: 2011-04-20
冒乃余
View PDF0 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0002] The existing traditional Chinese medicines for the treatment of allergic purpura generally use western medicines, etc., which are not effective for some patients, and some have certain side effects
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
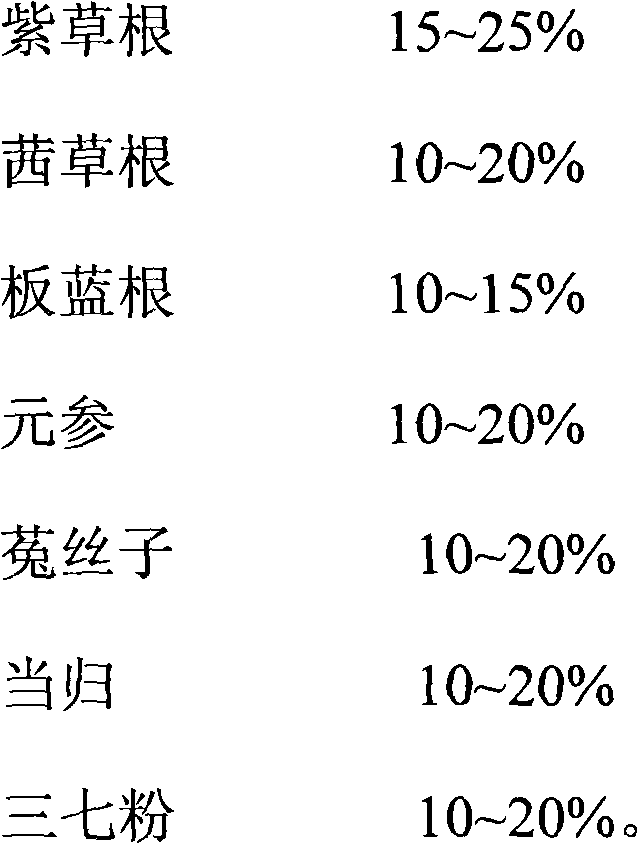
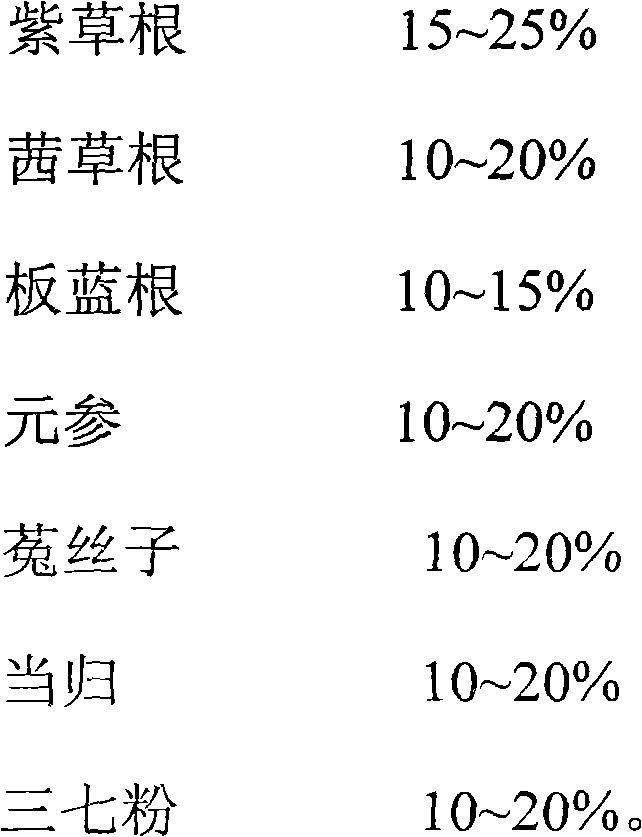
[0013] A medicine for treating allergic purpura, made of the following raw materials in weight percentage:
[0014]
[0015] When making, it is prepared according to the preparation method of conventional granules or tablets.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The present invention discloses a drug for treating allergic purpura, which is prepared from raw materials of lithospermum root, rubia cordifolia root, isatis root, scrophularia, cuscuta chinensis, angelica sinensis, and panax notoginseng powder. The present invention has good using effects, exact curative effects and a high effective rate of above 75%.
Description
Technical field: [0001] The invention relates to a traditional Chinese medicine for treating allergic purpura. Background technique: [0002] The existing traditional Chinese medicine for the treatment of Henoch-Schonlein Purpura generally adopts western medicine etc., which have poor curative effect on some patients, and some have certain side effects. Invention content: [0003] The object of the present invention is to provide a medicine for treating allergic purpura with good therapeutic effect. [0004] Technical solution of the present invention is: [0005] A medicine for treating allergic purpura is characterized in that it is made of the following raw materials in weight percentage: [0006] [0007] The medicine for the treatment of Henoch-Schonlein Purpura is made from the following raw materials in percentage by weight: [0008] [0009] [0010] The invention has good application effect, definite curative effect, and the effective rate is more than...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K36/74A61P37/08A61P7/04
Inventor 冒乃余
Owner 冒乃余
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com