Therapeutic for hepatic cancer
A liver cancer and antibody technology, applied in the direction of antibody medical components, drug combinations, peptides, etc., can solve the problems of hindering chemotherapy agents and exacerbation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0324] Effects of anti-glypican 3 antibody and chemotherapeutic agents (mitoxantrone or adriamycin hydrochloride) on mouse models transplanted with human liver cancer cell lines expressing glypican 3
[0325] (1) cell line
[0326] As human liver cancer cell lines expressing glypican 3, HuH-7 cells (Human Science Research Resource Bank) and HepG2 cells (ATCC) were used. HuH-7 cells were maintained and subcultured with Dulbecco's Modifid Eagle's Medium (SIGMA) containing 10% FBS (BIONET), and HepG2 cells were maintained and subcultured with sodium pyruvate (Invitrogen) containing 10% FBS, 1 mmol / l MEM, 1 mmol / l MEM was maintained and subcultured in Minimum Essential Medium Eagle medium (SIGMA) of non-essential amino acids (Invitrogen).
[0327] (2) Establishment of human liver cancer cell line transplanted mouse model
[0328] Each cell was prepared using a solution containing equal amounts of each subculture medium and MATRIGEL Matrix (BD Bioscience) so that it was 5×10 7 c...
Embodiment 2
[0345] Effects of humanized Glypican 3 antibody and chemotherapeutic agent (Sorafenib) on mouse models transplanted with human liver cancer cell lines expressing Glypican 3
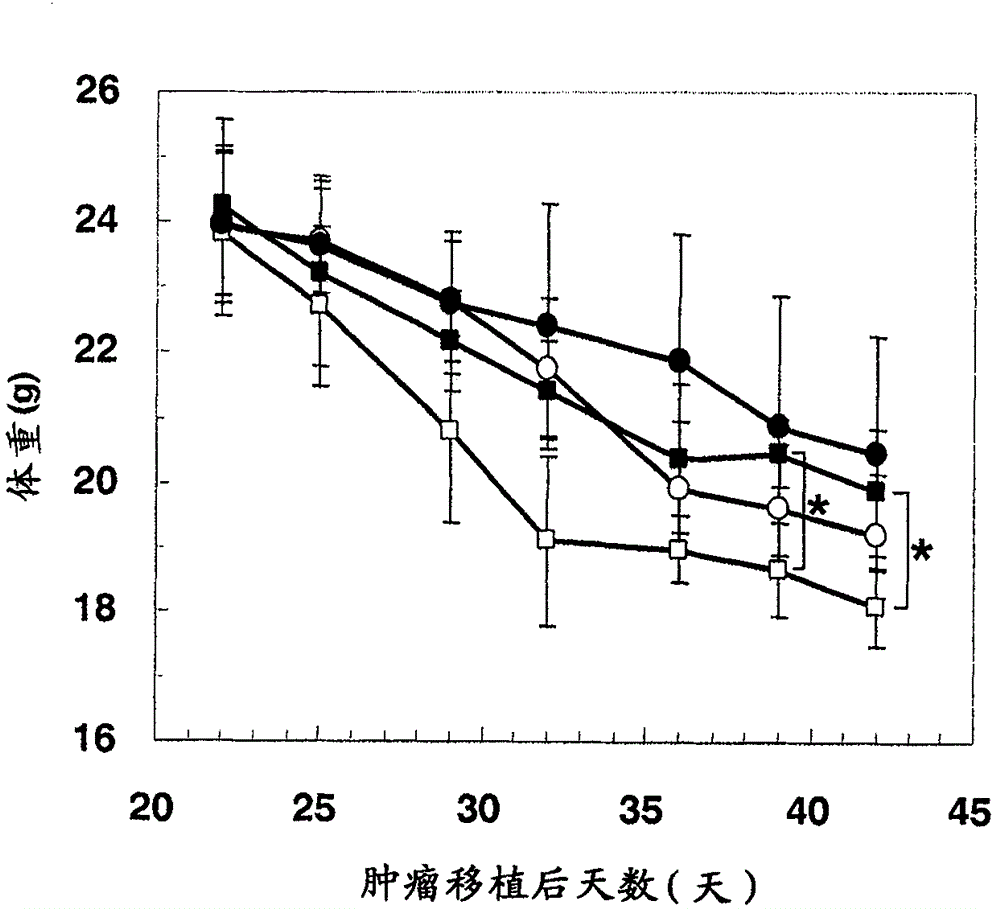
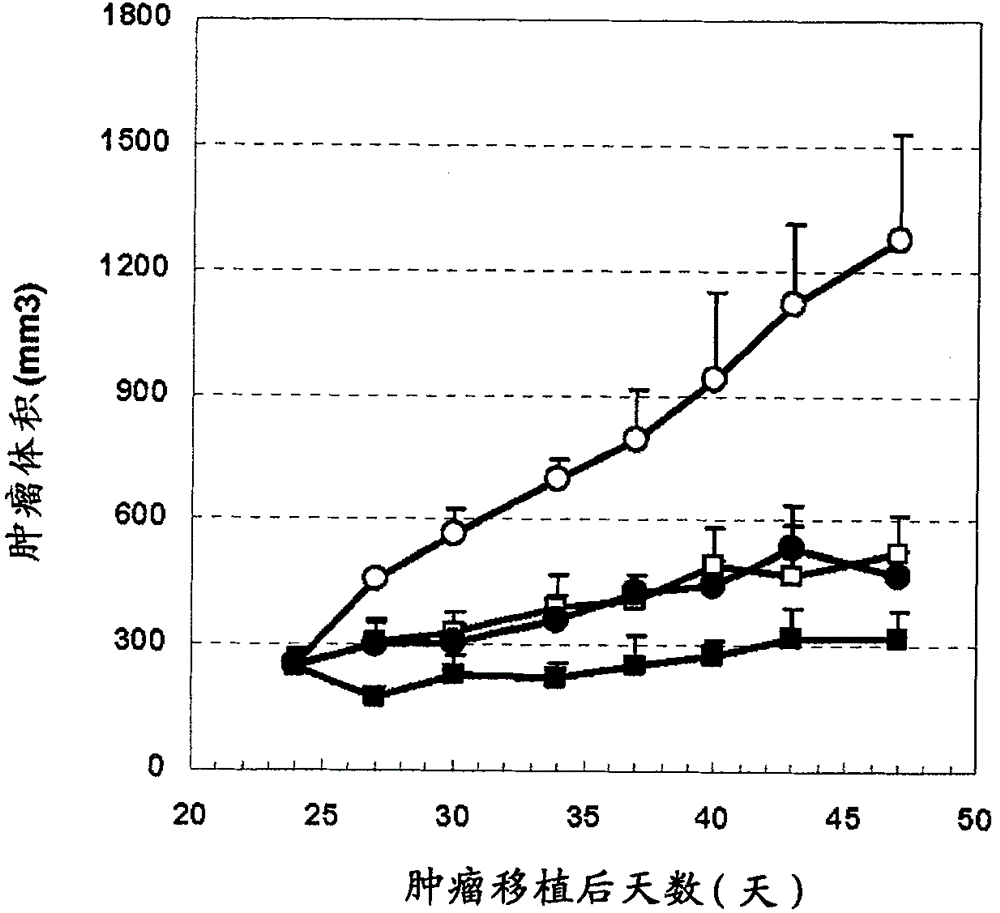
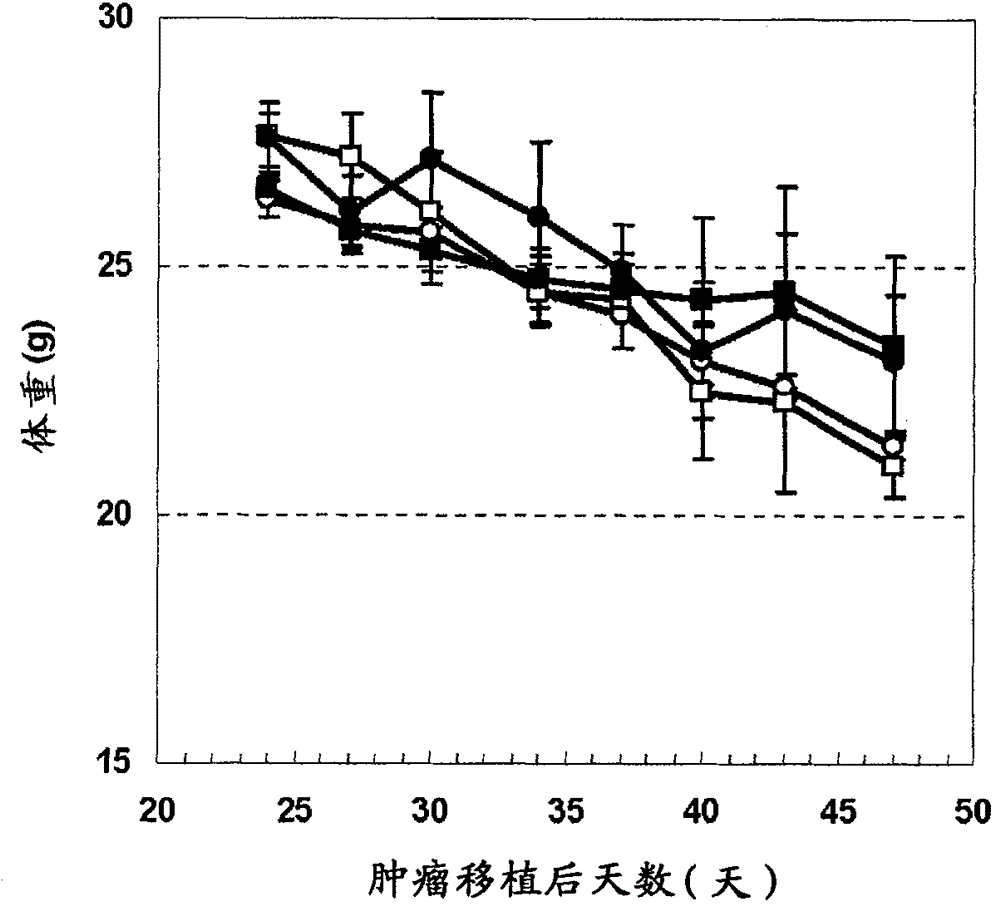
[0346] 6-week-old male CB-17 SCID mice were purchased from CLEA Japan Inc. Immediately before tumor transplantation, 200 μg of anti-asialo-GM1 antibody (WAKO) was intraperitoneally administered to mice, and then subcutaneously transplanted 5×10 5 A dispersion obtained by dispersing HepG2 cells or HuH-7 cells in 50% Matrigel (Becton Dickinson). When the tumor volume reaches 250mm 3 At the moment, the mice were divided into groups, and the administration was started. The humanized anti-glypican 3 antibody (hGC33, WO2006006693) was formulated with PBS (-) at an appropriate concentration and administered intravenously once a week for 3 weeks. Sorafenib was synthesized according to the method described in Organic Process Research & Development (2002) 6, 777-781, suspended in pure water containing 10% ethano...
Embodiment 5
[0391] (1) Combined use of humanized Glypican 3 antibody and chemotherapeutic agent (Sorafenib) on mouse models transplanted with human liver cancer cell lines expressing Glypican 3
[0392] 6-week-old male CB-17 SCID mice were purchased from CLEA Japan Inc. Immediately before tumor transplantation, 200 μg of anti-asialo-GM1 antibody (WAKO) was intraperitoneally administered to mice, and then subcutaneously transplanted 5×10 5 A dispersion obtained by dispersing HepG2 cells in 50% Matrigel (Becton Dickinson). When the tumor volume reaches 250mm 3 At the moment, the mice were divided into groups, and the administration was started. The pH7pL16 antibody was prepared at an appropriate concentration with PBS(-) and administered intravenously once a week for 3 weeks. Sorafenib was synthesized according to the method described in Organic Process Research & Development (2002) 6, 777-781, suspended in pure water containing 10% ethanol and 10% Cremophor EL, and administered orally ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com