Method for predicting tumor metastasis and invasion capacity in vitro and nucleotide fragments
A transfer ability, nucleotide technology, applied in biochemical equipment and methods, DNA/RNA fragments, microbial determination/inspection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
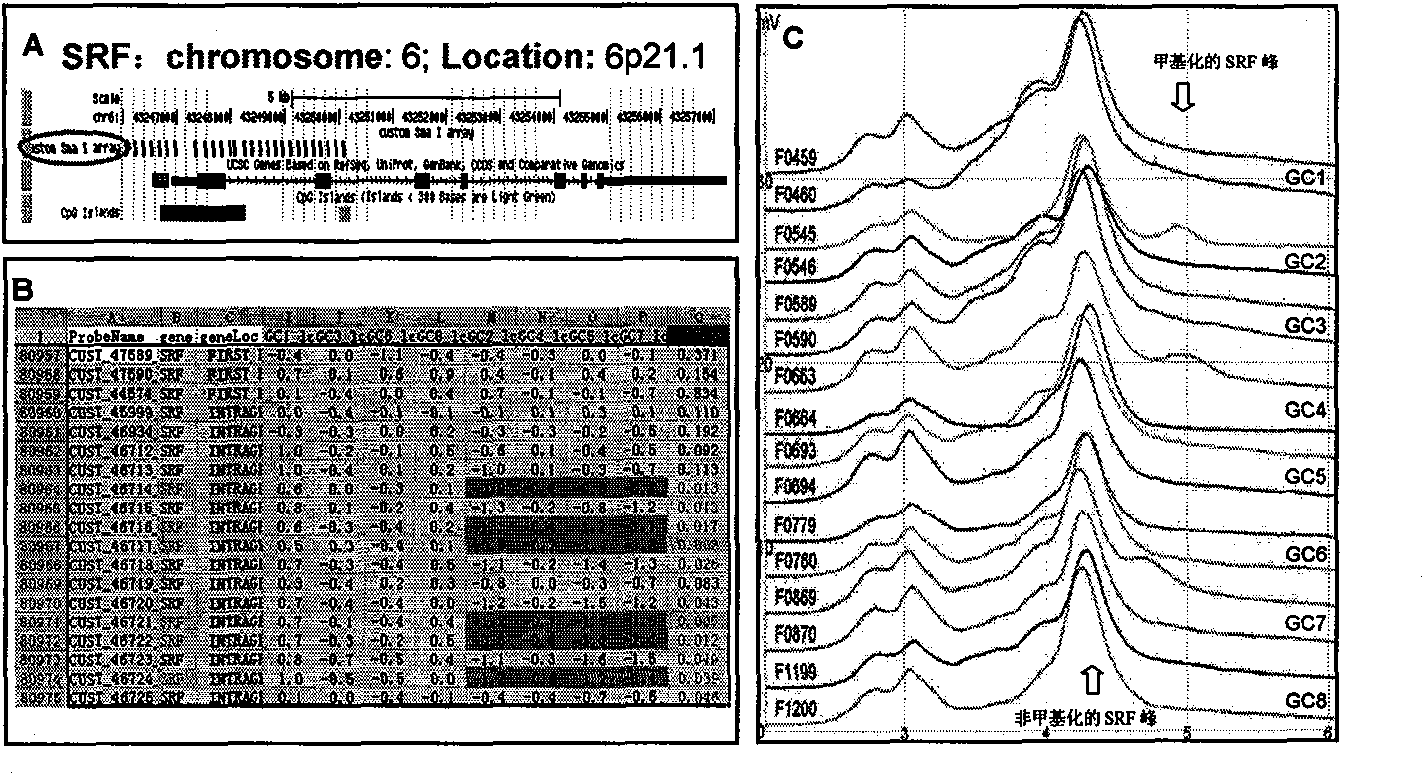
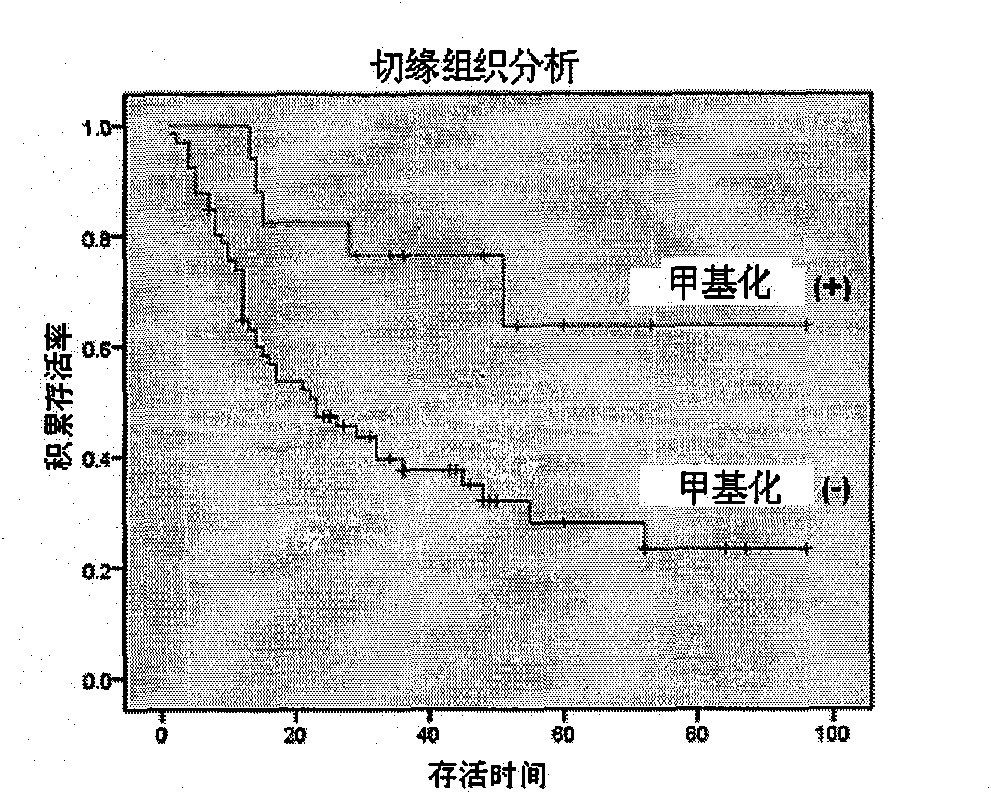
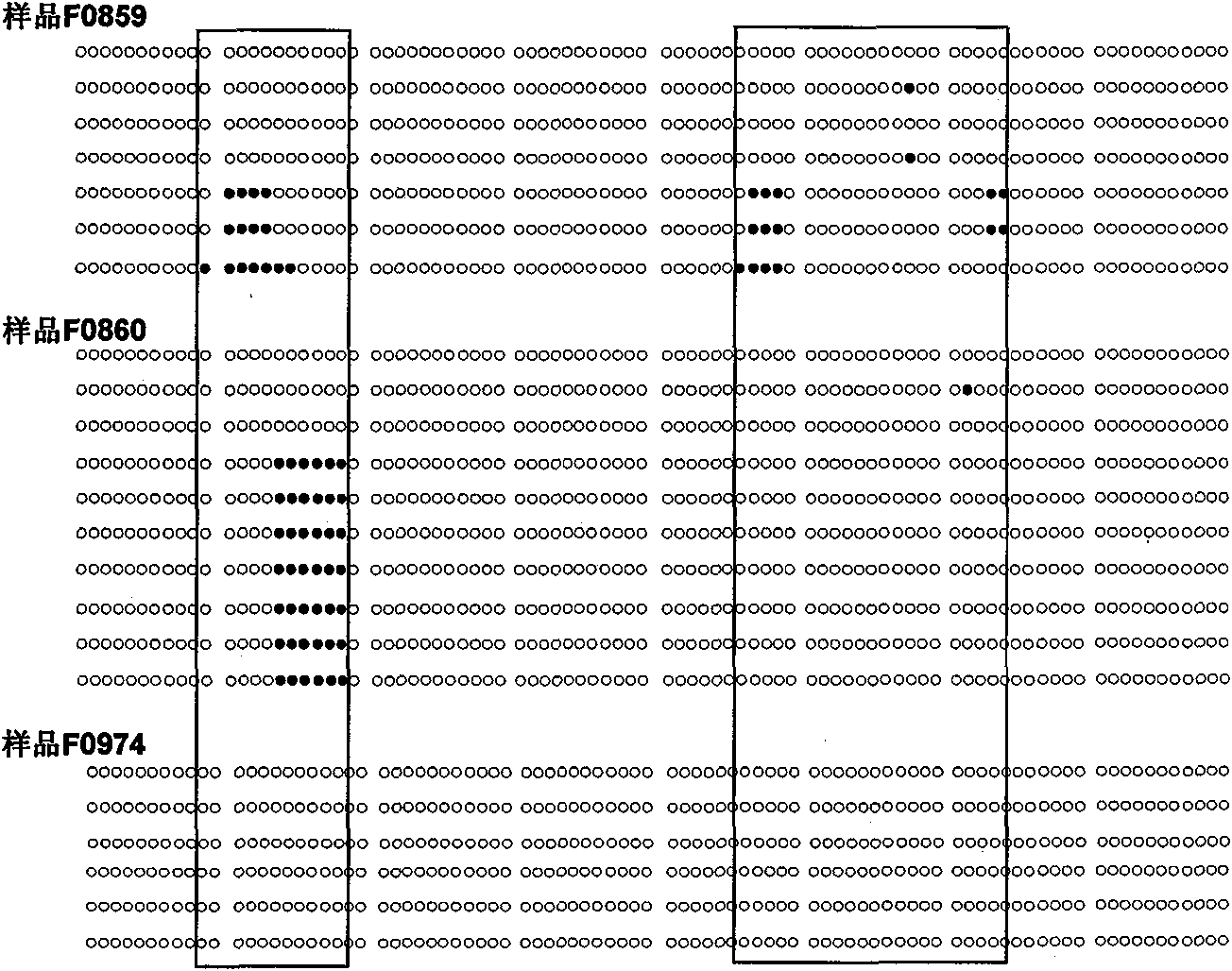
[0094] Example 1: Predicting the ability of gastric cancer invasion and metastasis by determining whether there is methylation of cytosine in the CpG island in the SRF nucleotide sequence in gastric cancer margin tissue
[0095]1. Experimental subjects: 102 paired frozen tissues of gastric cancer and their surgical margins, 50% of the patients had lymph node metastases and vascular tumor thrombi (or distant metastases), and 50% of the paired gastric cancer tissues had no lymph node metastasis No vascular tumor thrombus (or distant metastases) was seen, and complete clinicopathological data and follow-up data were available;
[0096] 2. Obtain tissue cells: cut out the size of rice grains (15-20 mm) from different parts of the surgical margin tissue 3 ) of five frozen tissues were collected into siliconized microcentrifuge tubes (1.5ml);
[0097] 3. Extract cell DNA: add 300μl tissue cell lysate (containing 10mM Tris.Cl / pH 7.6, 10mM NaCl, 10mM EDTA and 0.5% SDS) to the centrif...
Embodiment 2
[0114] Example 2: Predicting the ability of invasion and metastasis of thyroid cancer by determining whether there is methylation of cytosine in the CpG island in the SRF nucleotide sequence in the resection margin tissue of thyroid cancer
[0115] 1. Subjects: 20 pairs of thyroid cancer frozen tissues, 50% of the patients had lymph node metastasis and vascular tumor thrombus (or distant metastasis), and 50% of the paired patients had neither lymph node metastasis nor vascular cancer Thrombus (or distant metastases), all have complete clinicopathological data and follow-up data;
[0116] 2. Steps 2-7 are the same as in Example 1;
[0117] 3. Results ( Figure 4 ) and conclusion are the same as in Example 1.
Embodiment 3
[0118] Example 3: Prediction of gastric cancer invasion and metastasis by determining whether there is methylation of cytosine in CpG island in SRF nucleotide sequence in gastric cancer margin tissue
[0119] Steps 1-5 are the same as in Example 1; in step 6, except for determining whether there is a serum response factor gene DNA sequence whose number of methylated cytosines in the 5'-terminal CpG island is greater than or equal to 6, the rest are the same as in Example 1 Step 6 in is identical; The specificity of the measured result is higher than embodiment 1, is 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com