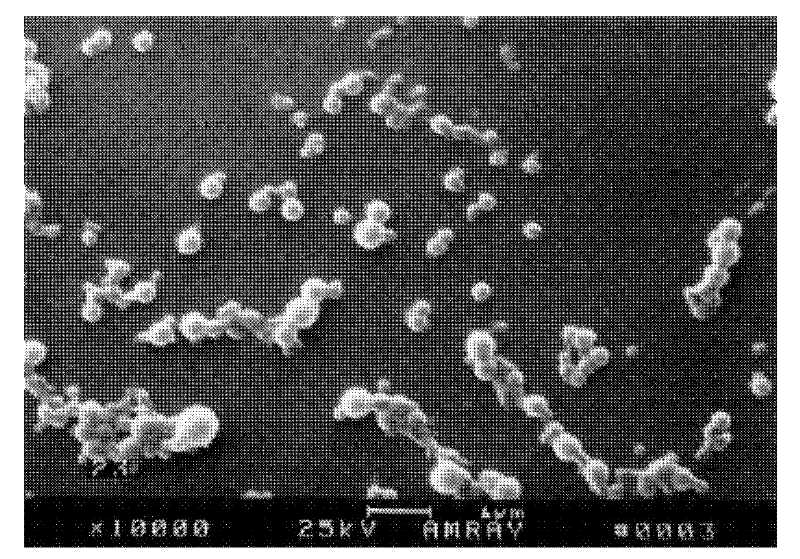
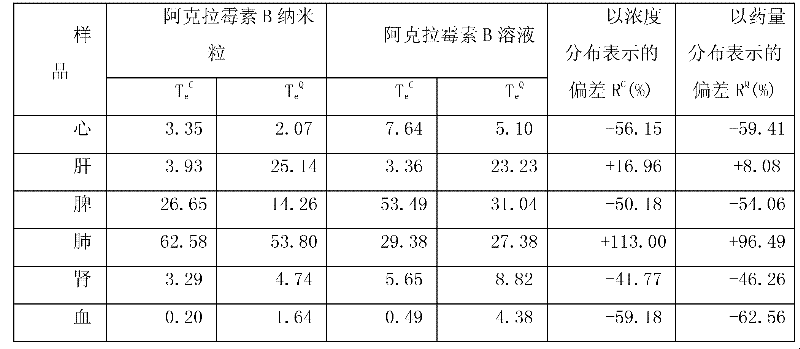
Aclacinomycin nanometer grain and preparation method thereof
A technology of aclarithromycin and nanoparticles, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc., and can solve problems such as high selectivity, secondary antigen-antibody reactions, and low drug loading , to increase the amount of drugs, eliminate toxicity and side effects, and improve the effect of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The preparation method of the aclarithromycin nanoparticles of the present embodiment comprises the following steps:
[0022] a. Take 1 volume part of organic solvent as the oil phase solution solvent, and dissolve 10% aclarmycin A or aclarmycin B in the organic solvent according to the total volume percentage of the oil phase; The content is 2.5% of phospholipids and bile salts, and the ratio of phospholipids to bile salts is 8:2. The phospholipids are dissolved in organic solvents to form an oil phase solution; wherein, the organic solvents are low-boiling organic solvents, such as methylene chloride, trichloro Methane, ethyl acetate, benzene or toluene; The present embodiment adopts dichloromethane, certainly adopt other low-boiling point and the organic solvent that can not cause damage to aclarithromycin can also achieve the purpose of the invention; Low-boiling point organic solvent is easy to follow-up process Evaporation in medium is conducive to the preparation...
Embodiment 2
[0028] The preparation method of the aclarithromycin nanoparticles of the present embodiment comprises the following steps:
[0029] a. Get 4 parts by volume of organic solvent as the oil phase solution solvent, and dissolve 2% aclarmycin A or aclarithromycin B in the organic solvent according to the total volume percentage of the oil phase; The content is 1.25% of phospholipid and bile salt, the ratio of phospholipid to bile salt is 1:4, and the phospholipid is dissolved in an organic solvent to form an oil phase solution; wherein, the organic solvent is a low-boiling organic solvent, such as dichloromethane, trichloro Methane, ethyl acetate, benzene or toluene; The present embodiment adopts chloroform, and of course adopts other low boiling point organic solvents that will not cause damage to aclarithromycin; the purpose of the invention can also be achieved; the low boiling point organic solvent is easy to follow-up process Evaporation in medium is conducive to the preparat...
Embodiment 3
[0034] The preparation method of the aclarithromycin nanoparticles of the present embodiment comprises the following steps:
[0035] a. Get 4 parts by volume of organic solvent as the oil phase solution solvent, and dissolve 14% aclarmycin A or aclarmycin B in the organic solvent according to the total volume percentage of the oil phase; The content is 6.25% of phospholipids and bile salts, and the ratio of phospholipids to bile salts is 1:4. The phospholipids are dissolved in an organic solvent to form an oil phase solution; wherein, the organic solvent is a low-boiling organic solvent, such as methylene chloride, trichloro Methane, ethyl acetate, benzene or toluene; The present embodiment adopts ethyl acetate, certainly adopt other low-boiling point and the organic solvent that can not cause damage to aclarithromycin can also achieve the purpose of the invention; Low-boiling point organic solvent is easy to follow-up process Evaporation in medium is conducive to the preparat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com