Centrum injection dilator and manufacture method thereof
A manufacturing method and technology of a dilator, which are applied in the field of medical devices, can solve the problems of complicated manufacturing, leakage of biological materials, and difficulty in grasping the timing of extraction of braided wires, and achieve the effect of simple structure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment approach
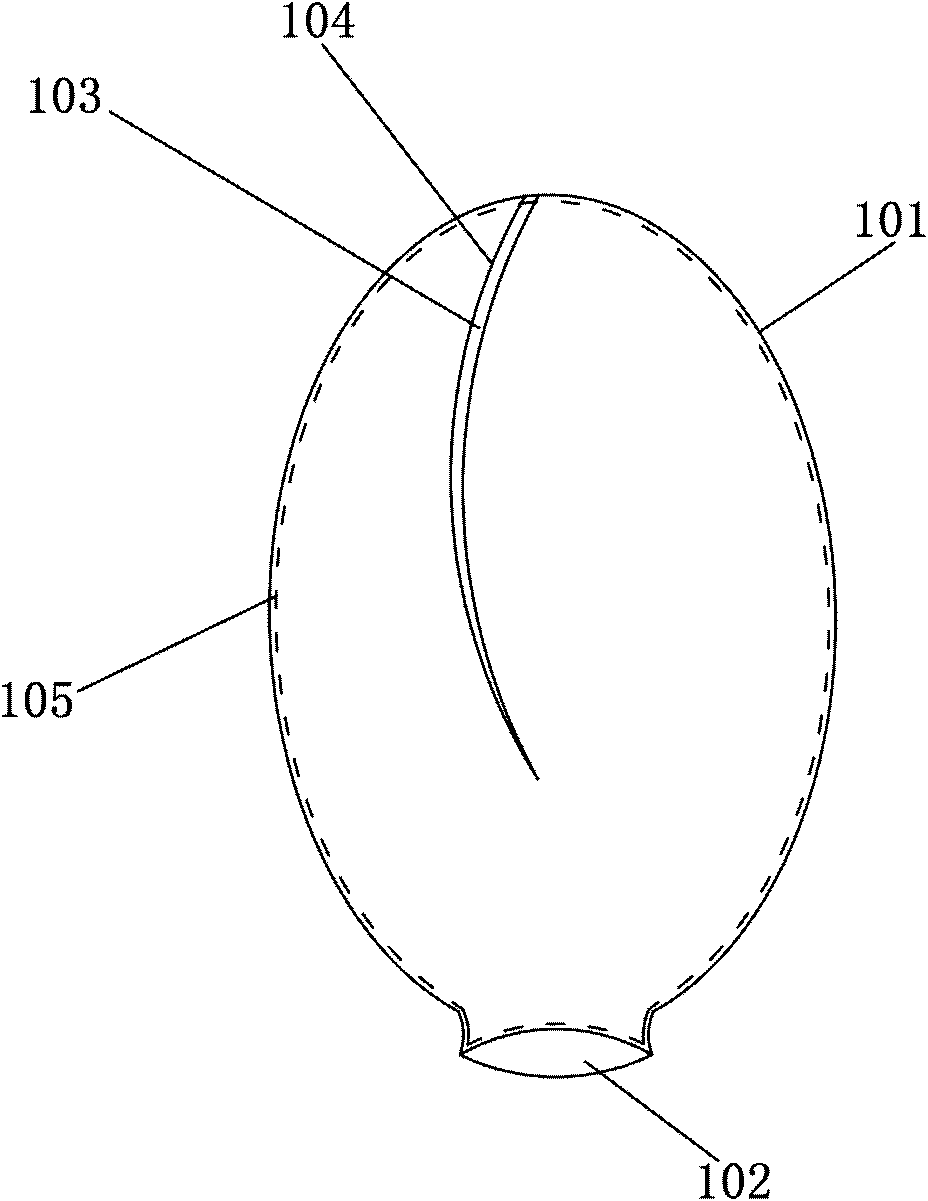
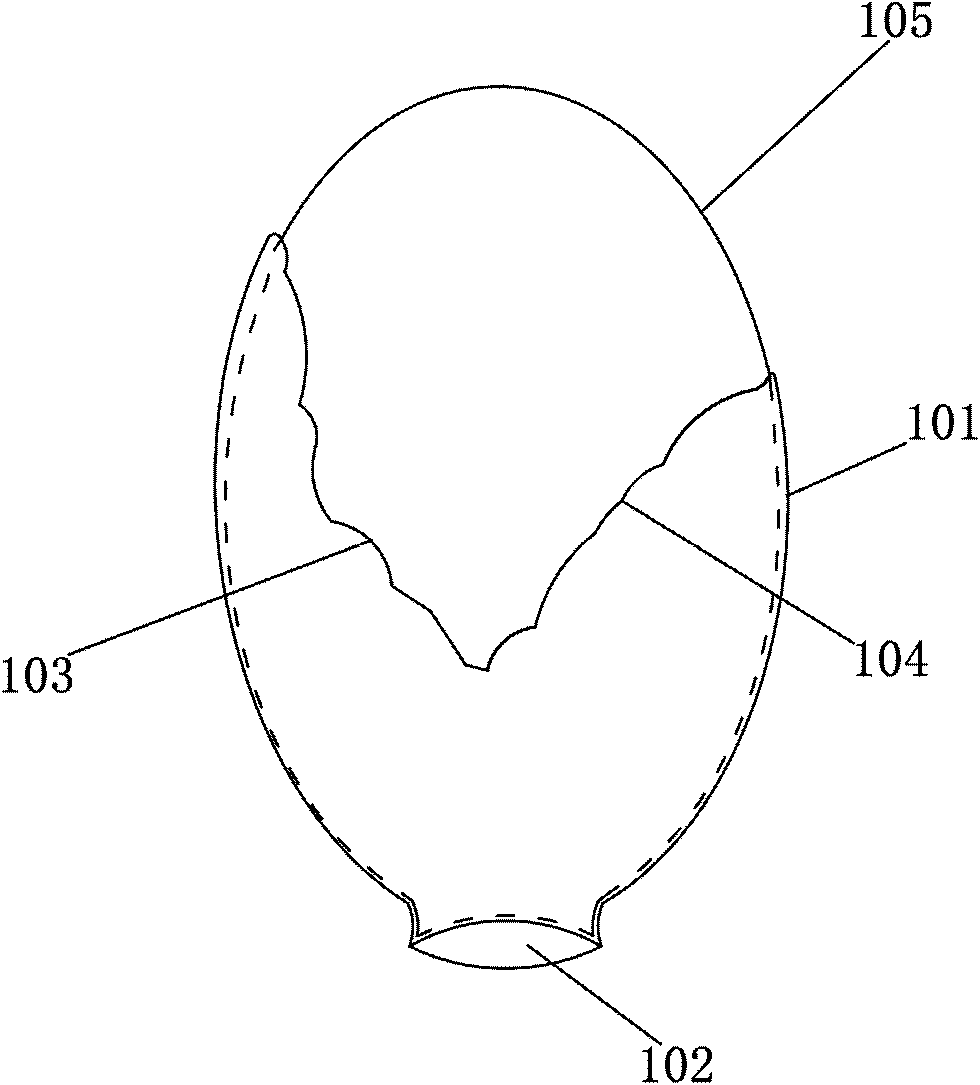
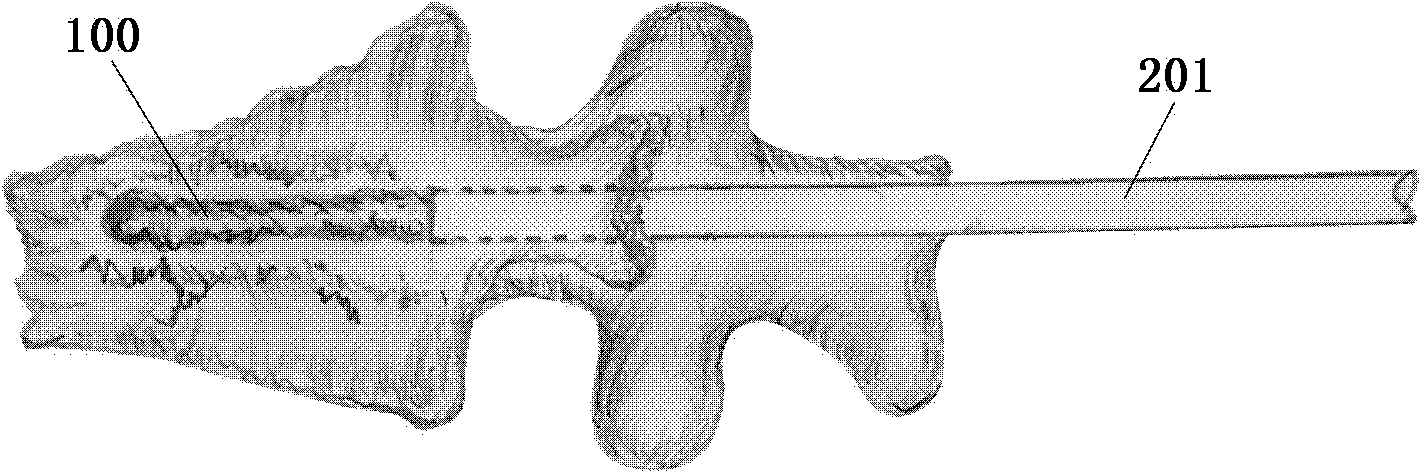
[0063] According to one embodiment of the manufacturing method of the vertebral body injection dilator of the present invention, the manufacturing method comprises the following steps:
[0064] A balloon 100 having a balloon body 101 and an infusion port 102 is formed. Wherein, an opening 103 is formed on the balloon body 101, and the size of the opening 103 is set to allow the solidification 105 of the biological material in the balloon 100 to escape through the opening 103;
[0065] The opening 103 is closed by an adhesive material 104 to form a complete balloon shape. Wherein, the adhesive material 104 has a softening point of a predetermined temperature value within an exothermic temperature range during the exothermic curing process of the biological material.
[0066] In one embodiment of the manufacturing method of the present invention, the balloon body is provided in the form of a sheet, and the opening 103 is formed on the sheet. The structure after its formation c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Softening point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com