Monoclonal antibody, composition taking same as active ingredient, and application of monoclonal antibody
A monoclonal antibody and composition technology, applied in the field of biology, achieves the effects of small injection volume, high degree of humanization, and small molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0017] The following examples of the present invention are presented for the purpose of illustration and not to limit the scope of the present invention.
[0018] Example 1. The production method of the monoclonal antibody of the present invention
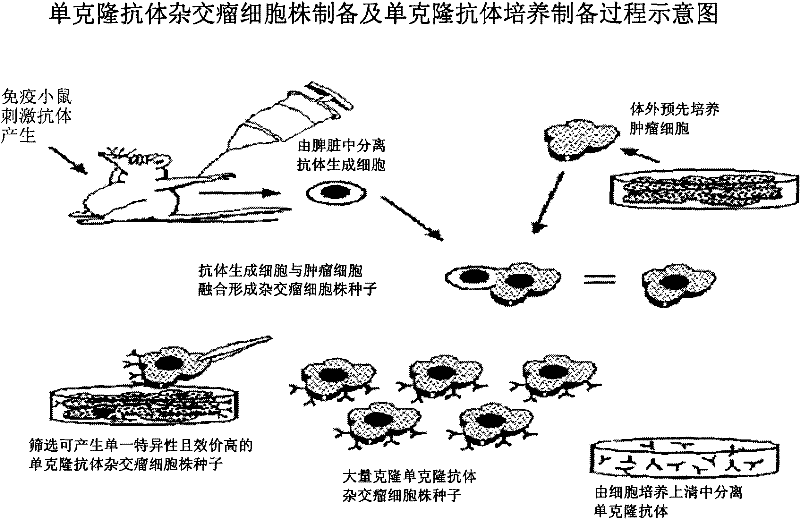
[0019] Monoclonal antibody is produced by monoclonal method, which is a popular and practical process for preparing monoclonal antibody. Georges J.F. Kohler and Cesar Milstein first prepared monoclonal antibodies in the world in 1975. They won the Nobel Prize in Medicine and Physiology in 1984 because they proposed a method for preparing monoclonal antibodies.
[0020] Scientists have long known how to make antibodies. They inject antigens (such as viruses and tumor cells) into animals. B cells in the animal's immune system will release antibody molecules (molecules that specifically bind to antigens) in the blood. However, it is almost impossible to extract the required pure antibodies in batches from serum. Experiments usi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com