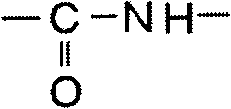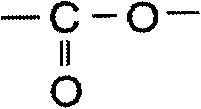Electrophotographic toner and production method of the same
A manufacturing method and toner technology, which can be used in developing agents, instruments, electrographics, etc., and can solve problems such as low strength and carrier contamination.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example (1
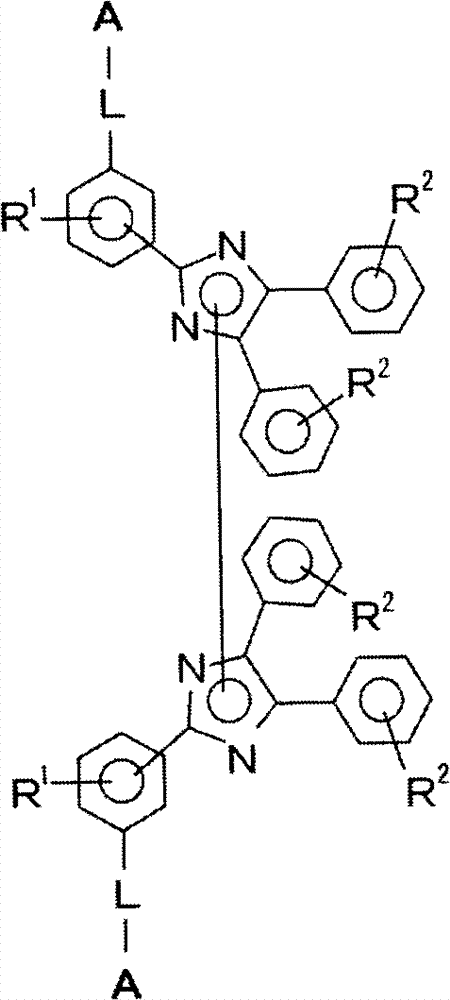
[0156] First, in the first synthesis process, an unmodified polyester resin as a raw material polyester prepolymer and a triphenylimidazole compound represented by formula (2-1) as a specific triphenylimidazole compound are reacted, Thus, the carboxyl group of the unmodified polyester resin and the amino group of the triphenylimidazole compound represented by the formula (2-1) are bonded by reaction, thereby forming a connection composed of a composite group of the carboxyl group and the amino group. The site, that is, the connection site having the structure represented by formula (a), thereby introducing the triphenylimidazole group into the raw polyester prepolymer, and synthesizing the prepolymer into which the triphenylimidazole group was introduced.
[0157] Next, in the second synthesis process, the imidazole rings of the prepolymers into which triphenylimidazole groups have been introduced are subjected to a reaction treatment in which the imidazole rings are bonded, wh...
Synthetic example (2
[0159] In the first synthesis process, the amino-modified polyester resin as the raw material polyester prepolymer and the triphenylimidazole compound represented by the formula (2-4) as the specific triphenylimidazole compound are reacted, thereby , the amino group of the amino-modified polyester resin and the carboxyl group of the triphenylimidazole compound represented by the formula (2-4) are bonded by reaction, thereby forming a linking site with a structure represented by the formula (a), and the A triphenylimidazole group was introduced into a raw material polyester prepolymer to synthesize a triphenylimidazole group-introduced prepolymer.
[0160] Next, in the second synthesis process, a reaction treatment is performed to bond the imidazole rings between the triphenylimidazole group-introduced prepolymers, whereby the two triphenylimidazole group-introduced prepolymers pass through The polyester resin in which L in the general formula (1) is a group represented by the ...
Synthetic example (3
[0162] In the first synthesis process, the unmodified polyester resin as the raw material polyester prepolymer and the triphenylimidazole compound represented by the formula (2-2) as the specific triphenylimidazole compound are reacted to avoid The carboxyl group of the modified polyester resin and the hydroxyl group of the triphenylimidazole compound represented by the formula (2-2) are bonded by reaction, thereby forming a linking site composed of a composite group of the carboxyl group and the hydroxyl group, i.e. The linking site having the structure represented by the formula (b) introduced the triphenylimidazole group into the raw polyester prepolymer, and synthesized the triphenylimidazole group-introduced prepolymer.
[0163] Next, in the second synthesis process, a reaction treatment is performed to bond the imidazole rings between the triphenylimidazole group-introduced prepolymers, whereby the two triphenylimidazole group-introduced prepolymers pass through A specif...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com