Sequential and repeated application of four or more HIV vector gene vaccines
A carrier vaccine and vaccine technology, applied in the field of biomedicine, can solve the problem of restricting the repeated application of vaccines, and achieve the effect of long-term maintenance of high level, good specific immune response, and prolonged time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
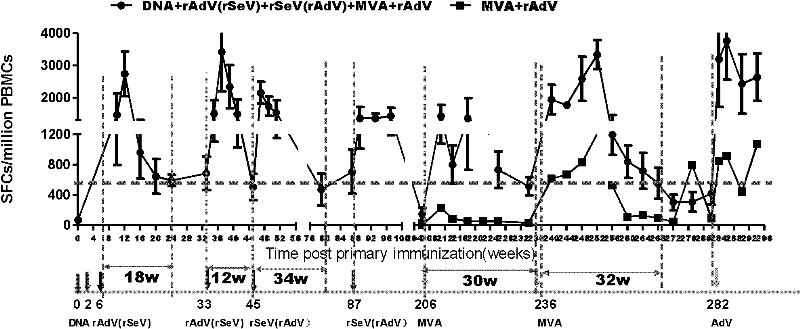
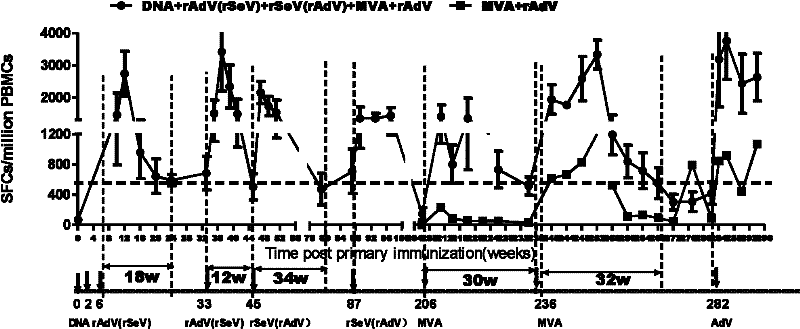
[0019] HIV vaccine sequential and repeated immunization experiment in rhesus monkeys
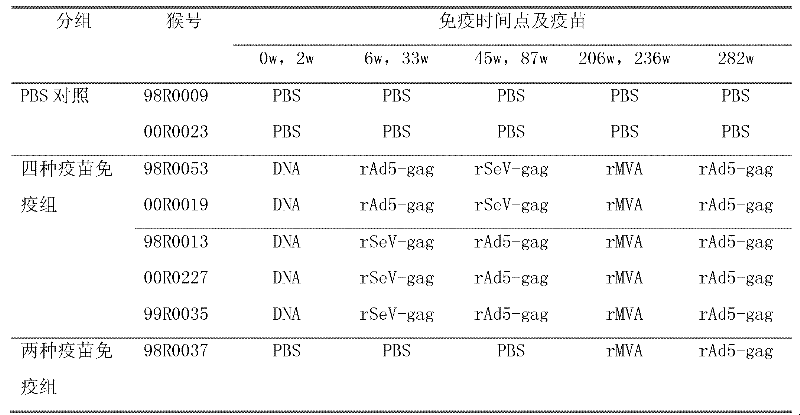
[0020] 1. Vaccination and grouping
[0021] Eight adult rhesus monkeys were randomly divided into 3 groups. Group 1 and 2 were PBS control groups. At all the same immunization time points as the experimental group, 1ml sterile PBS was injected intramuscularly; Group 2 5 were four vaccine-immunized groups , First use DNA in 0 weeks and immunize twice in 2 weeks (The DNA vaccine used is a eukaryotic expression plasmid expressing the codon-optimized gag gene, which is a prior patent application filed by the applicant in 2006 with the application number 200610098946.1 The constructed pVR-mod.gag, produced by Benyuan Zhengyang Gene Technology Co., Ltd.), was immunized with the same dose of adenovirus vaccine rAd5-gag (rAd5-gag is E1, which expresses mod.gag gene) at 6 and 33 weeks. The replication-defective recombinant type 5 adenovirus with E3 deletion, the expressed promoter is mCMV, and the tailin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com