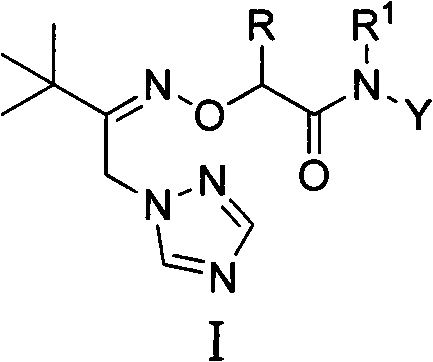
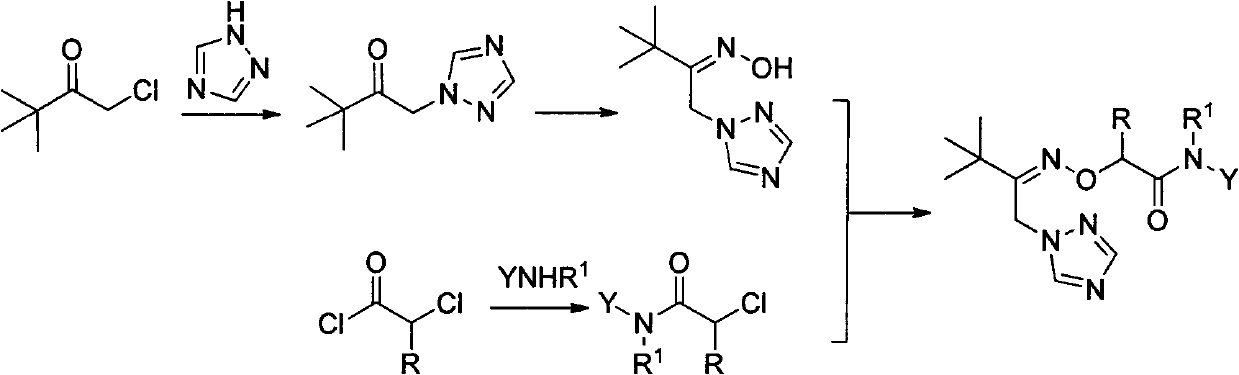
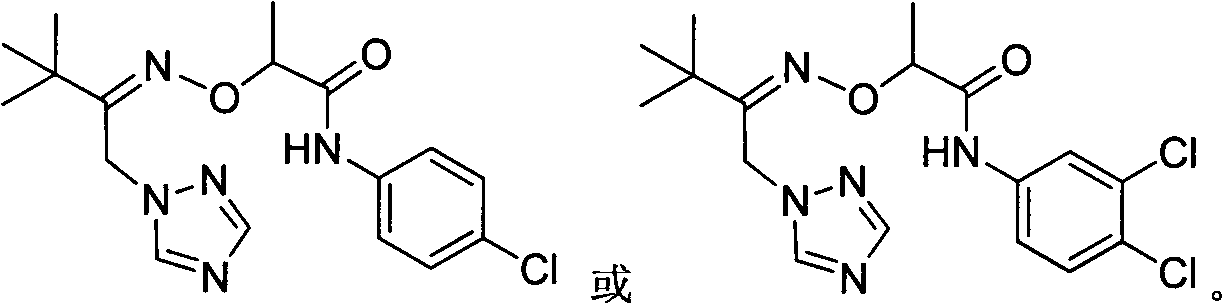
1-(1,2,4-Triazolyl) Ketoxime Ether Amide and Its Application
A technology of ketoxime ether amide and triazole base, which is applied in the field of new compounds and their preparation, and can solve the problems of no reports of herbicidal and insecticidal activities.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 N-benzyl-2-[3,3-dimethyl-1-(1,2,4-triazol-1-yl)butyl-2-methyleneaminooxy]acetamide (4a ) preparation
[0034]
[0035] 2.16g (20.2mmol) benzylamine, 1.95g (14.1mmol) potassium carbonate, 1.73g (2.88mmol) PEG600, 20mL dichloromethane, N 2 For protection, 3.02 g (27.0 mmol) of chloroacetyl chloride was slowly added dropwise under ice bath conditions, and the dropwise addition was completed in 30 minutes, and the reaction was tracked by TLC for 6.9 hours. Rotary evaporation, washing with water, drying, and ethanol recrystallization gave 2.67 g of white solid 3a, yield 71.9%, m.p.88-90°C.
[0036] 0.92g (5.0mmol) compound 2, 0.86g (4.96mmol) N-benzyl-2-chloroacetamide, 0.15g (0.5mmol) tetrabutylammonium bromide (TBAB), 0.08g (0.05mmol) KI, 15 mL of toluene. 1.98g of 20% sodium hydroxide (9.9mmol) solution was slowly added dropwise, and the dropwise addition was completed within 10min, and the temperature was slowly raised to 60°C, and the reaction was comple...
Embodiment 2
[0037] Example 2 N-(3-methylphenyl)-2-[3,3-dimethyl-1-(1,2,4-triazol-1-yl)butyl-2-methyleneamine oxide The preparation of base] acetamide (4b)
[0038]
[0039] Prepare 3b according to Example 1, react for 3.9h, and obtain 2.32g white solid after recrystallization from ethanol, yield 62.7%, m.p.86~87°C; prepare compound 4b according to Example 1, react for 2.8h, yield 24.2% , m.p.101-102°C. 1 H NMR (CDCl 3 , 400Hz) δ: 1.14(s, 9H, 3×CH 3 ), 2.37 (s, 3H, CH 3 ), 2.27 (s, 3H, CH 3 ), 4.73 (s, 2H, CH 2 ), 5.09 (s, 2H, NOCH 2 ), 6.97 (d, J=7.6Hz, 1H, C 6 h 4 4-H), 7.25(t, J=8.4Hz, 1H, C 6 h 4 5-H), 7.37(d, J=8.4Hz, 1H, C 6 h 4 6-H), 7.47(s, 1H, C 6 h 4 2-H), 7.94(s, 1H, C 2 h 2 N 3 3-H), 8.23(s, 1H, C 2 h 2 N 3 5-H), 9.19 (s, 1H, CONH).
Embodiment 3
[0040] Example 3 N-(3,4-dimethylphenyl)-2-[3,3-dimethyl-1-(1,2,4-triazol-1-yl)butyl-2-ylidene Preparation of methylaminooxy]acetamide (4c)
[0041]
[0042] Prepare 3c according to Example 1, react for 1.5h, and obtain 2.75g off-white solid after ethanol recrystallization, yield 69.2%, m.p.94~96°C; prepare compound 4c according to Example 1, react for 1.4h, yield 49.5% , m.p.121-123°C. 1 H NMR (CDCl 3 , 400Hz) δ: 1.14(s, 9H, 3×CH 3 ), 2.24(s, 3H, CH 3 ), 2.27 (s, 3H, CH 3 ), 4.73 (s, 2H, CH 2 ), 5.09 (s, 2H, NOCH 2 ), 7.11 (d, J=7.6Hz, 1H, C 6 h 3 5-H), 7.30 (dd, J 1 =8.0Hz,J 2 = 2.4Hz, 1H, C 6 h 3 6-H), 7.40(s, 1H, C 6 h 3 2-H), 7.93(s, 1H, C 2 h 2 N 3 3-H), 8.20(s, 1H, C 2 h 2 N 3 5-H), 9.15 (s, 1H, CONH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com