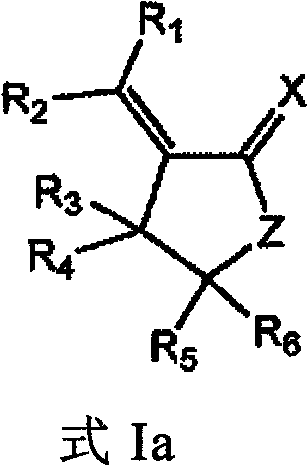
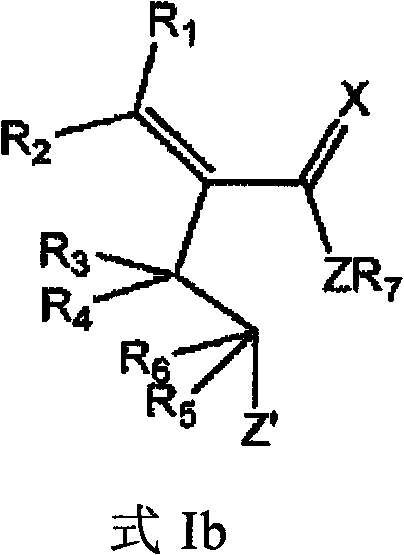
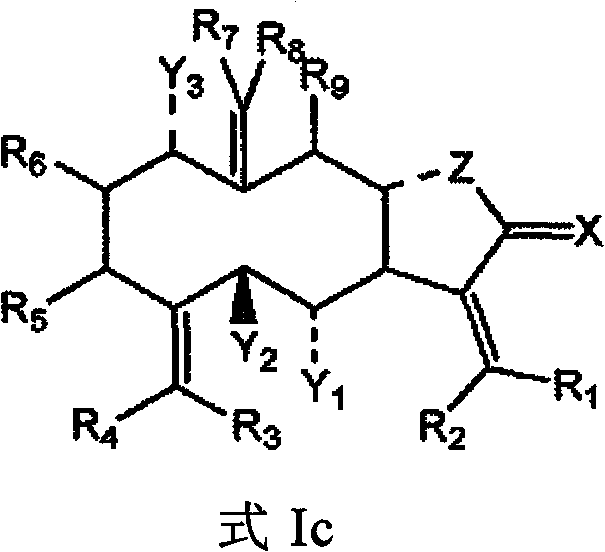
Method for screening compounds that selectively interact with rad9
A technology of compounds and complexes, applied in the fields of biochemical equipment and methods, determination/inspection of microorganisms, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0085] Example 1: Bioanalysis on Saccharomyces cerevisiae using Secroder
[0086] Cycloderm (LMSV-6) at concentrations between 10 μg / ml and 100 μg / ml on solid medium against a panel of S. cerevisiae mutant strains whose DNA is similar to that found in tumors / cancer cells biological analysis. Cycloderm showed significant cytotoxic activity only against mutant strains, especially the gene rad9. This suggests that Secroderma is selectively cytotoxic to cells with unstable genomes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com