Reaction method and reactor for preparing acrylic acid through selective oxidation of propane
A reactor, acrylic acid technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of decreased selectivity of acrylic acid, conversion of propylene into acrylic acid, large differences, etc., to reduce resource consumption and save Energy consumption, effect of highly selective conversion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
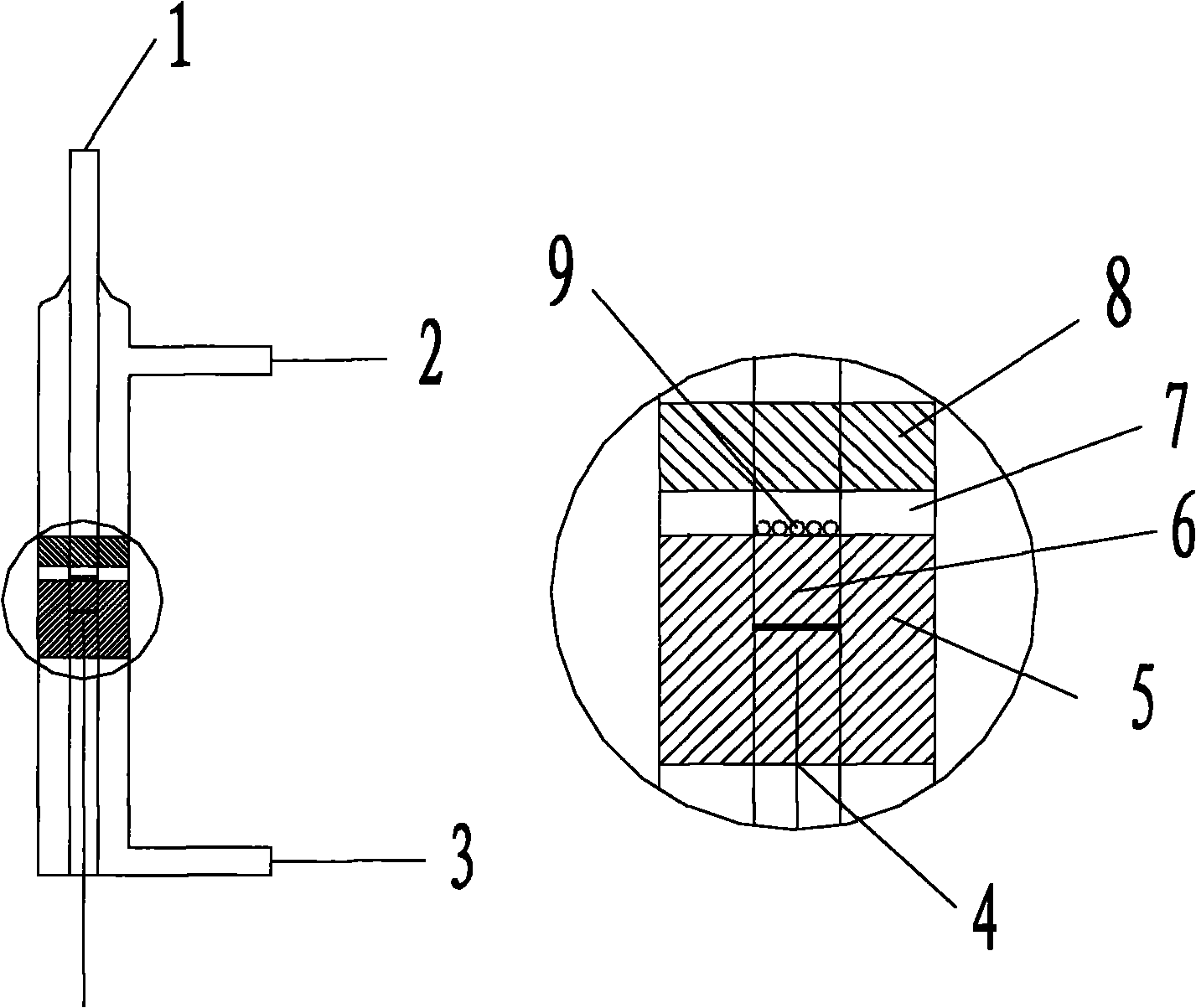
[0032] In a common fixed-bed reactor, catalyst 1 uses a nickel-zirconium oxide catalyst for the oxidative dehydrogenation of propane; catalyst 2 uses a molybdenum-vanadium oxide catalyst for the selective oxidation of propylene. The mass ratio of catalyst 1 and catalyst 2 is 2:3. Quartz sand with a mass ratio of 0.2 to 1:1 of the catalyst 1 is contained between the two layers of catalysts. The reaction temperature is 340° C., and the reaction pressure is normal pressure. In the feed gas, the molar ratio of propane to oxygen is 6.6:5.4, and does not contain distributed oxygen and water vapor.
Embodiment 2
[0034] In the cross-sectional oxygen distribution reactor of the present invention, the reaction conditions are the same as in Example 1, and the molar ratio of propane to oxygen is 6.6:5.4. Wherein, the molar ratio of mixed oxygen and distributed oxygen is 6:1.
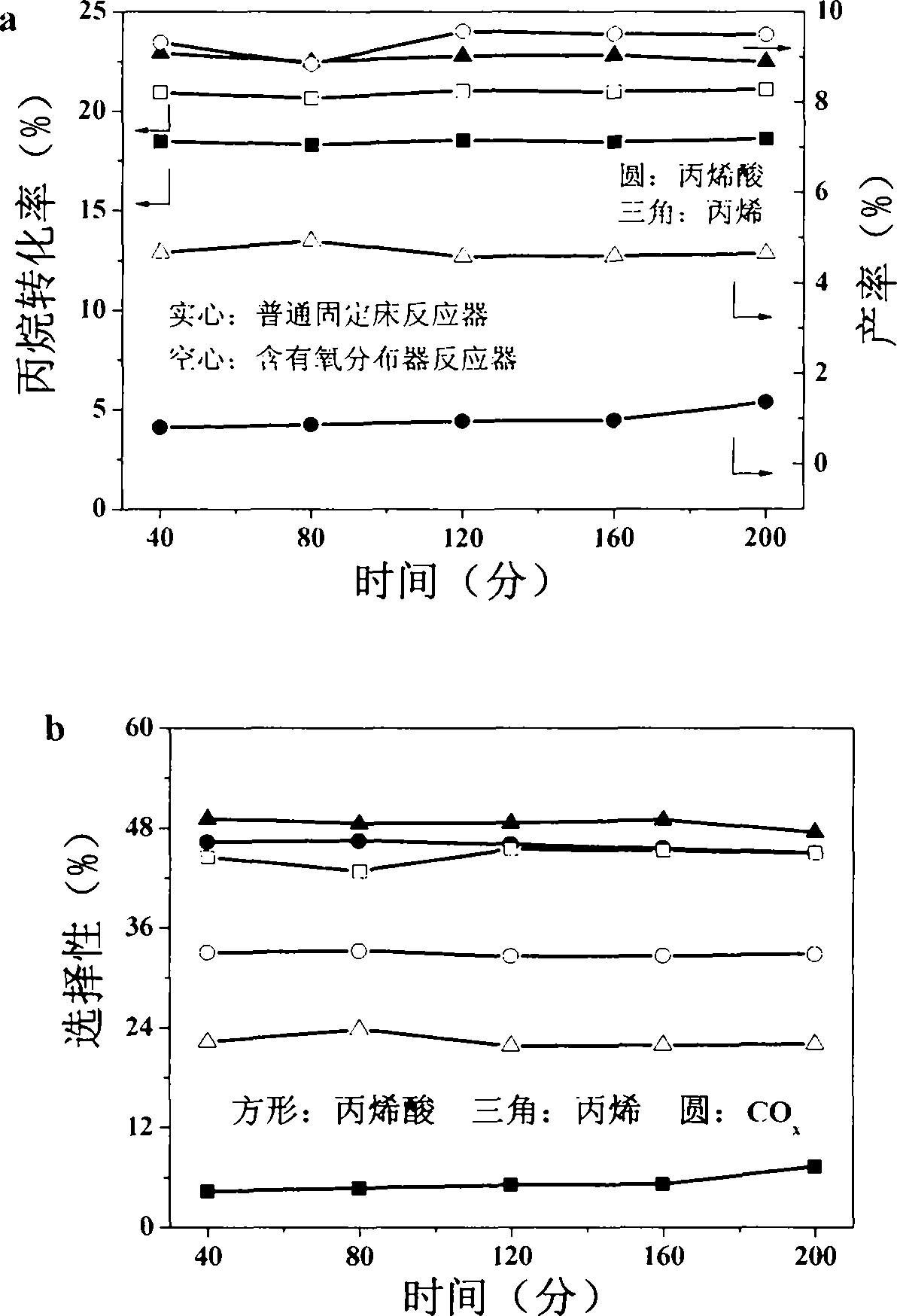
[0035] The reaction result of the above process is shown in figure 2 , through the comparison of the above two processes, using the reactor of the present invention, the performance of the selective oxidation of propane to acrylic acid has been greatly improved, not only the conversion rate has increased slightly, from 18% to 22%, and the selectivity of acrylic acid is obvious Improve, from less than 10% to 45%. At the same time, the selectivity of the by-product carbon dioxide decreased by more than 10%.
Embodiment 3
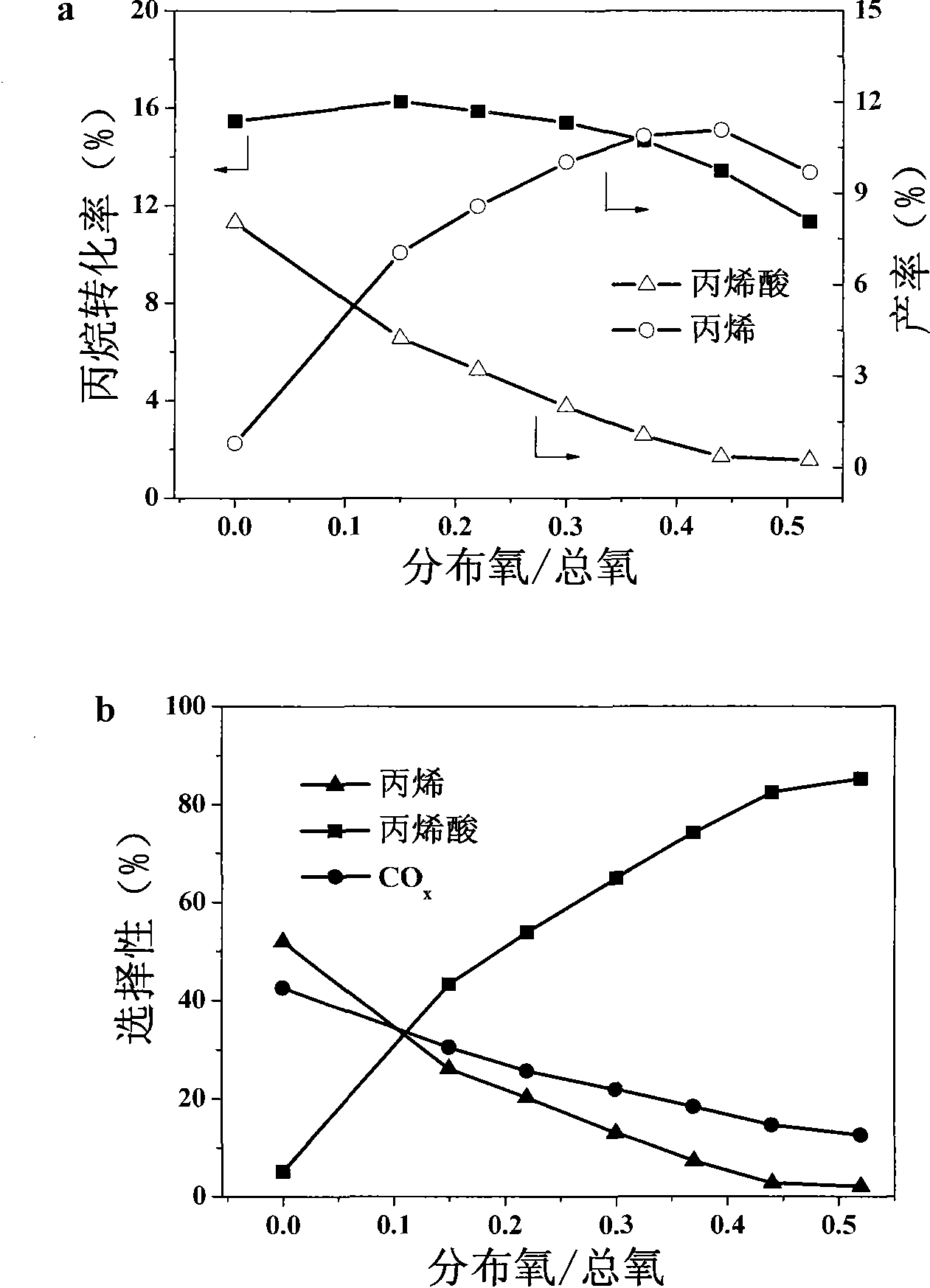
[0037] In the cross-sectional oxygen distribution reactor of the present invention, the mass ratio of the filled catalyst 1 to the catalyst 2 is 2:3, and the two layers of catalysts contain quartz sand with a mass ratio of 0.2 to 1:1 to the catalyst 1. The reaction temperature is 340° C., and the reaction pressure is normal pressure. In the feed gas, the molar ratio of propane to oxygen is 6.6:5.4, and there is no water vapor. The effect of different mixed oxygen and distributed oxygen feed ratios on the reaction performance is shown in image 3 .
[0038] The ratio of mixed oxygen and distributed oxygen has a great influence on the reaction result, changing the molar ratio means changing the alkoxygen ratio of the two processes. Depend on image 3 It can be seen that the conversion of propane reaches the maximum when the molar ratio of distributed oxygen to mixed oxygen is 0.15, and the best yield of acrylic acid is achieved when the molar ratio of distributed oxygen to mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com