Stabilized Fc polypeptides with reduced effector function and methods of use
A stable, number-specified technology, applied to medical preparations containing active ingredients, specific peptides, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0624] Example 1. Rational design of stability-enhancing IgG Fc mutations
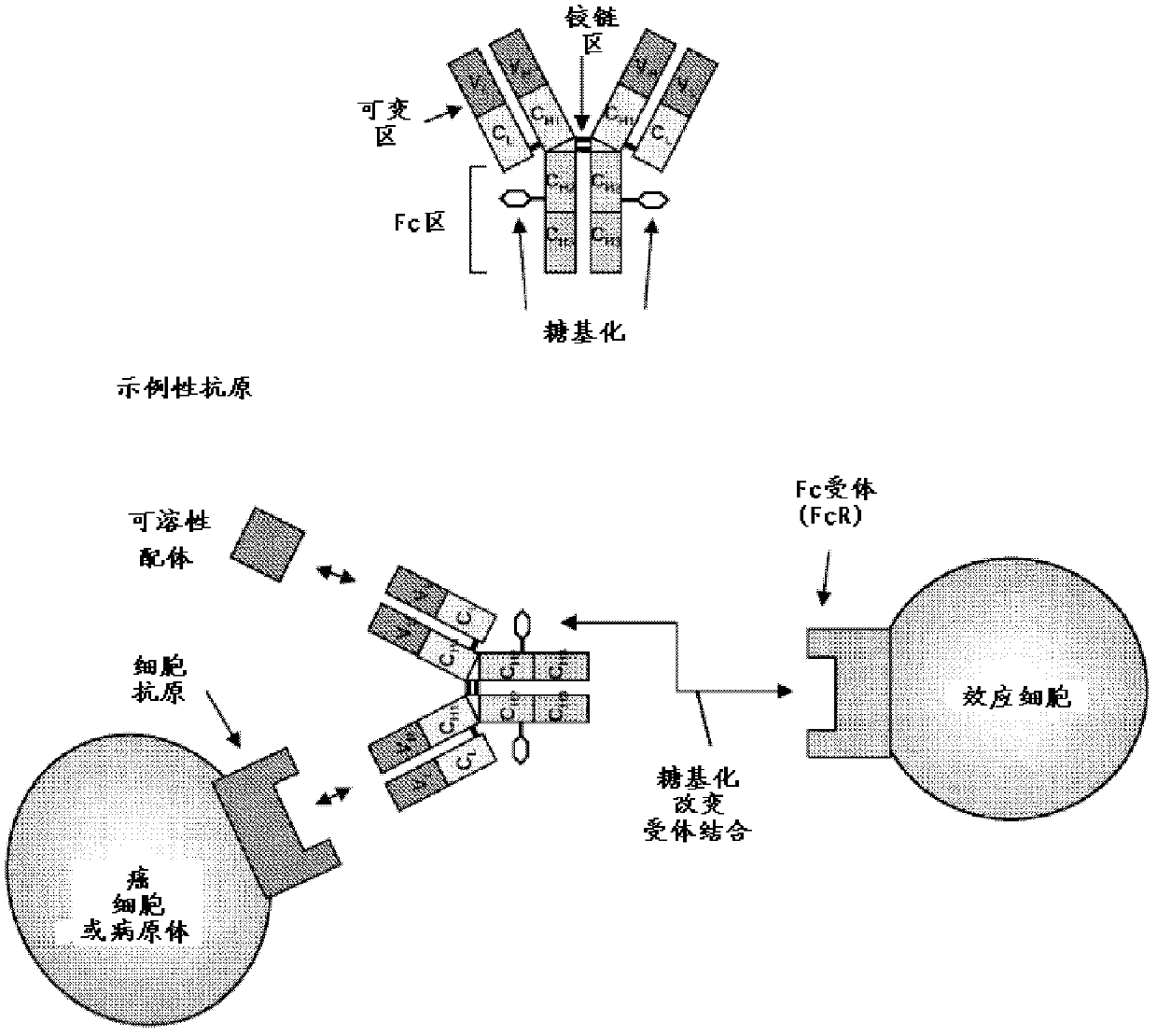
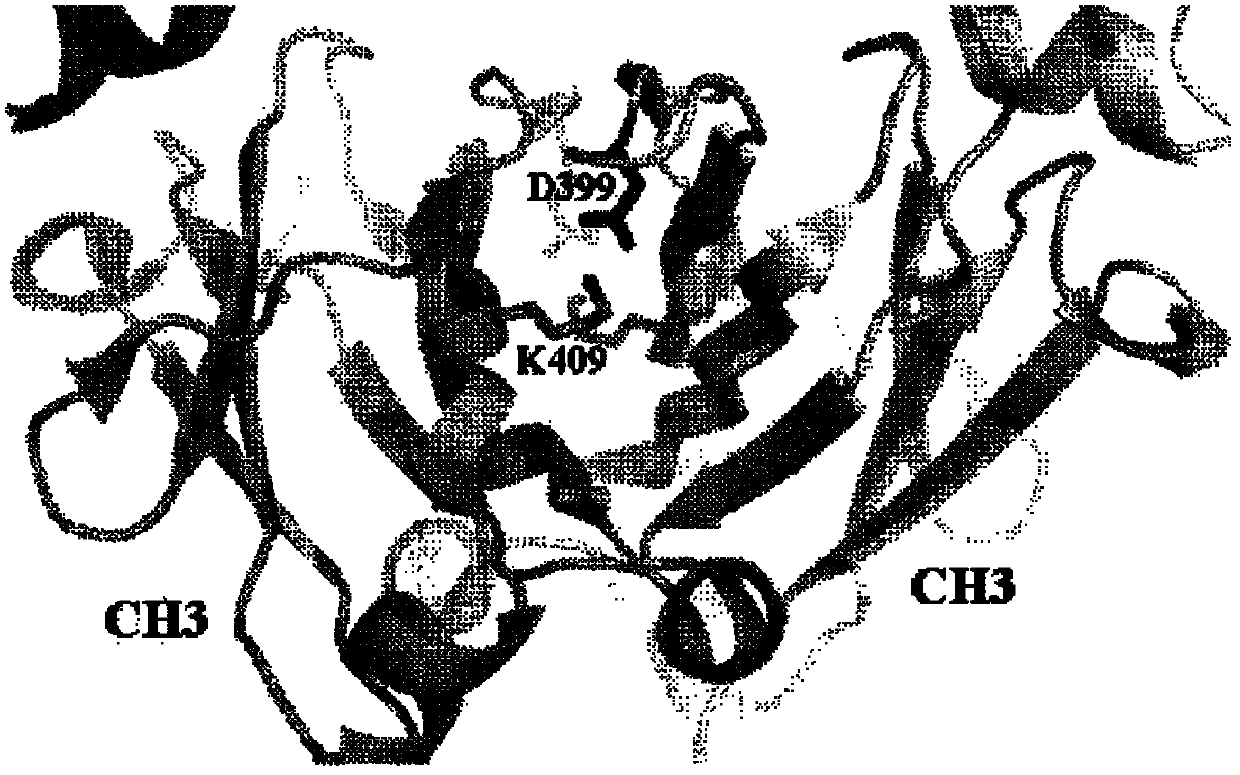
[0625] Aglycosylated antibodies represent an important class of therapeutic agents that do not require immune effector functions. However, it is clear that removal of CH2-associated oligosaccharides in IgG1 and IgG4 affects antibody conformation and stability. Loss of antibody stability can present process development challenges that adversely affect program timelines and resources. Here, we detail several methods for designing repertoires of amino acid positions in CH2 and CH3 to enhance IgG Fc stability.
[0626] A. Effector-less IgG: Associated Variation and Residue Frequency Design for the IgG4CH2 Domain
[0627] Correlative variation analysis was performed on different Cl class Ig fold sequence databases as previously described (Glaser et al., 2007; Wang et al., 2008). The compilation and structure / HMM-based alignment of the Cl class Ig fold sequences were also performed as previously describ...
Embodiment 2
[0657] Example 2. Thermostability screening of IgG Fc antibody domains produced in E. coli
[0658]A modified heat challenge assay described in US Patent Application Serial No. 11 / 725,970 was employed as a stability screen to determine the amount of soluble IgG Fc protein retained at 40°C after heat challenge at pH 4.5.
[0659] Escherichia coli strain W3110 (ATCC, Manassas, Va. Cat. #27325). Transformants were incubated at 30°C in SB (Teknova, Half Moon Bay, Ca.Cat.#S0140) supplemented with 0.6% glycine, 0.6% Triton X100, 0.02% arabinose and 50 μg / ml carbenicillin. Grow overnight in expression medium. Bacteria are pelleted by centrifugation and the supernatant collected for further processing.
[0660] After heat challenge, aggregated material was removed by centrifugation, and binding of soluble Fc samples retained in the treated clarified supernatants to protein A (delta P7837) was determined by DELFIA assay. Coat two 96-well plates (MaxiSorp, Nalge Nunc, Rochester, N...
Embodiment 3
[0665] Example 3. Production of stable IgG Fc antibodies
[0666] A. Mutagenesis, transient expression and purification of stable IgG Fc part in E. coli
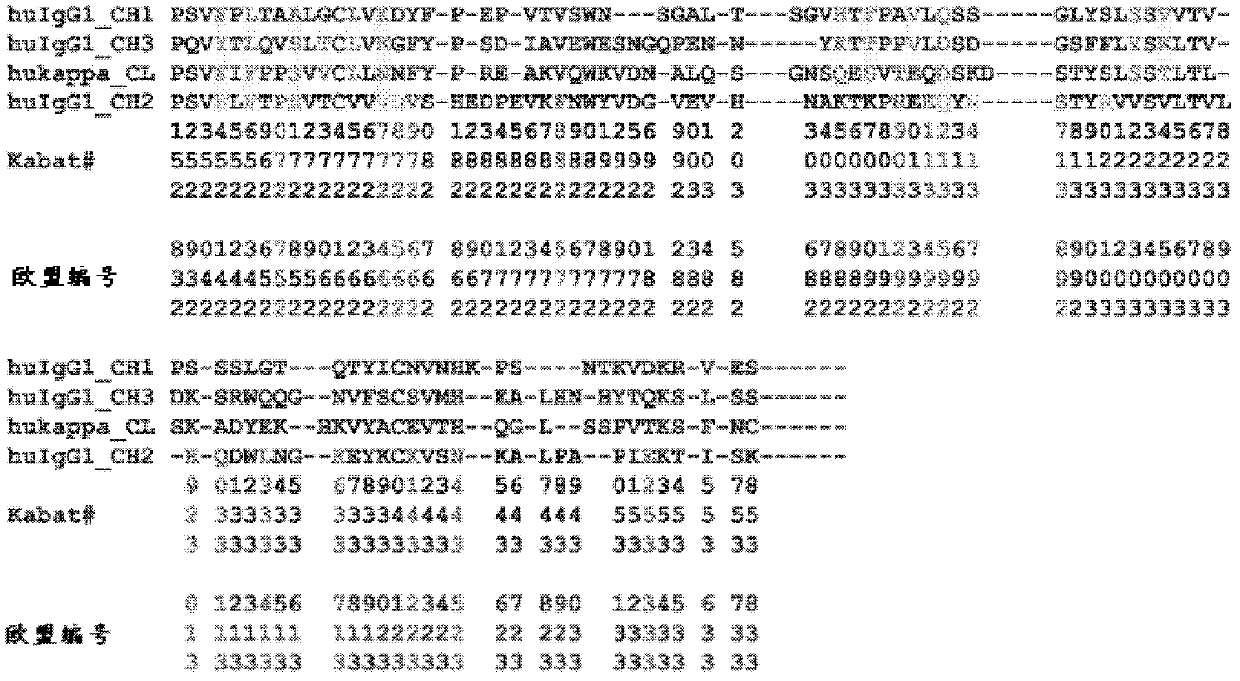
[0667] Stability mutations were incorporated into the BRM13 construct previously detailed in Example 2 by site-directed mutagenesis using the Stratagene Quik-Change Lightning Mutagenesis Kit. Primers were designed to be 36-40 bases in length, with mutations in the appropriate sequence 10-15 bases flanking the middle, with a GC content of at least 40%, starting and ending at one or more C / G bases . All mutant constructs are listed in Table 3.1 below.
[0668] Table 3.1. IgG-Fc constructs expressed and purified from E. coli
[0669]
[0670]
[0671] After PCR using primers to introduce mutations, mutagenesis was digested with DpnI restriction endonuclease for 5 minutes at 37°C to completely digest the parental plasmid. Then the mutagenesis reaction was transferred to XL1 blue E. coli supercompetent cells. Ampicil...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com