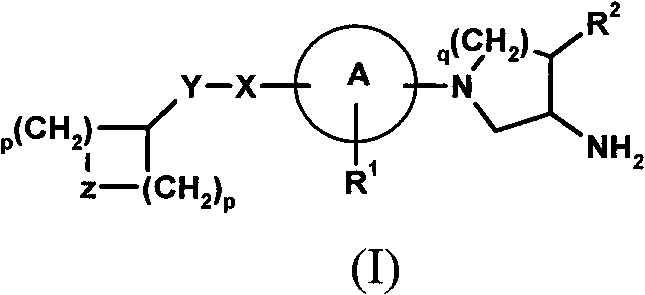
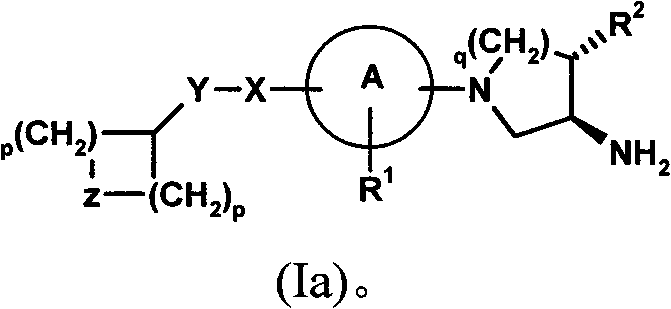
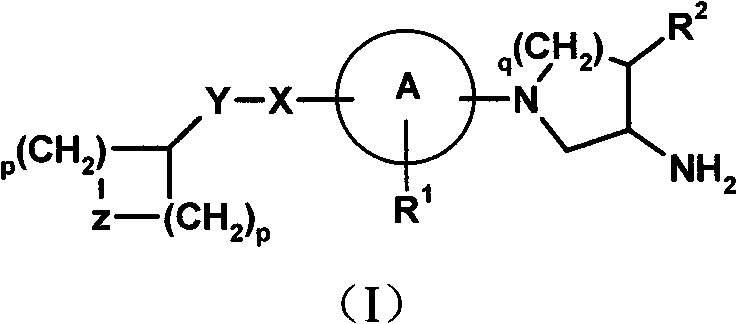
Compounds for the treatment of metabolic disorders
A compound, a technique of stereochemistry, applied in the field of therapeutic compounds for metabolic diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0160] Materials and methods
[0161] Unless otherwise specified, in SiO 2 (40-63 mesh) for column chromatography. LCMS data were acquired as follows: Atlantis 3 μC 18 Chromatography column (3.0×20.0mm, flow rate=0.85mL / min) with 0.1% HCO 2 H of H 2 O-MeCN solution was eluted for 6 minutes and detected by UV at 220 nm. Gradient information: 0.0-0.3min 100%H 2 O; 0.3-4.25min: Ramp up to 10% H 2 O-90% MeCN; 4.25-4.4min: ramp up to 100% MeCN; 4.4-4.9min: keep at 100% MeCN; 4.9-6.0min: return to 100% H 2 O. Using an electrospray ion source in positive (ES + ) or negative (ES - ) mass spectra were acquired in ion mode.
[0162] LCMS-method 2 data acquisition is as follows: Xbridge C18 column (2.1 × 50mm, 2.5μM, flow rate 0.8mL / min) with MeCN-10mM NH 4 HCO 3 The solution was eluted in 1.5 minutes with UV detection at 215-350 nm. Gradient information: 0-0.8min: 98% MeCN 2% NH 4 HCO 3 to 98% NH 4 HCO 3 2% MeCN; 0.8-1.2min: keep at 98% NH 4 HCO 3 2% MeCN. Using an...
Embodiment 1
[0491]Example 1: 1-[(3S,4S)-4-amino-1-(5-{(R)-3-[1-(5-chloropyrimidin-2-yl)piperidin-4-yl]butyl Oxy}pyrimidin-2-yl)pyrrolidin-3-yl]piperidin-2-one
[0492]
[0493] (R)-5-Chloro-2-{4-[1-methyl-3-(2-chloropyrimidin-5-yloxy)-propyl]piperidin-1-yl}pyrimidine (Preparation 4, 160 mg, 0.42 mmol), tert-butyl [(3S,4S)-4-(2-oxopiperidin-1-yl)pyrrolidin-3-yl]carbamate (Preparation 41, 148 mg, 0.53 mmol) and DBU (160 mg, 1.05 mmol) in DMSO (2 mL) was heated to 100° C. for 16 hours. The mixture was diluted with water, then the organics were extracted into DCM (×3) and dried (MgSO 4 ). The solvent was removed in vacuo followed by purification by column chromatography (IH:IPA, 100:0, 85:15) to give [(3S,4S)-1-(5-{(R)-3-[1-(5-chloro tert-butyl carbamate : RT=4.67min; m / z (ES + )=629.3[M+H] + . The residue was dissolved in DCM (5 mL), then TFA (1 mL) was added before the mixture was stirred for 20 min. with saturated Na 2 CO 3 The solution was quenched and the organics were extr...
Embodiment 2
[0494] Example 2: 1-[(3S,4S)-4-amino-1-(5-{(R)-3-[1-(3-isopropyl-[1,2,4]oxadiazole- 5-yl)piperidin-4-yl]butoxy}pyridin-2-yl)pyrrolidin-3-yl]piperidin-2-one p-toluenesulfonate
[0495]
[0496] To (R)-2-bromo-5-{3-[1-(3-isopropyl-[1,2,4]-oxadiazol-5-yl)piperidin-4-yl]butoxy } To a solution of pyridine (preparation 5, 200 mg, 0.47 mmol) in dioxane (5 mL) was added [(3S,4S)-4-(2-oxopiperidin-1-yl)pyrrolidin-3-yl] tert-butyl carbamate (Preparation 41, 160 mg, 0.56 mmol), 2,8,9-triisobutyl-2,5,8,9-tetraaza-1-phosphabicyclo-[3.3.3]deca Monoalkane (16.2 mg, 0.05 mmol), potassium tert-butoxide (159 mg, 1.65 mmol) and tris(dibenzylideneacetone)dipalladium (43 mg, 0.05 mmol). Argon was bubbled through the mixture for 30 minutes, then heated at 120° C. for 60 minutes in a microwave reactor. The mixture was diluted with DCM, followed by saturated NaHCO 3 solution, washed with brine, and dried (MgSO 4 ). The solvent was removed in vacuo and the residue was purified by column chroma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com