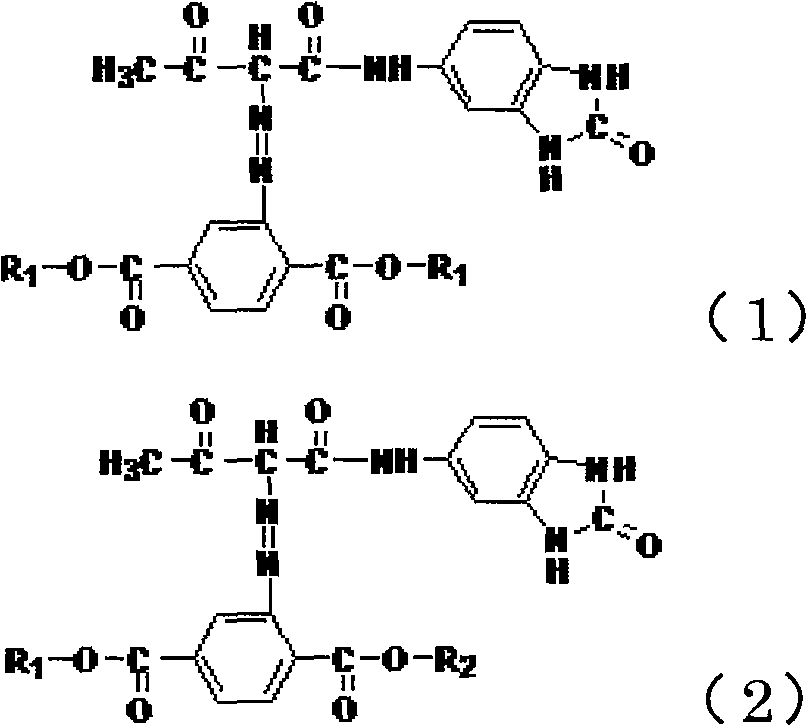
High-strength yellow azo mixed pigment
A yellow azo, pigment technology, applied in the field of dyes, can solve the problems of low strength, hindering mass use, etc., and achieve the effect of high coloring strength, good brightness and high transparency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0050] According to a second aspect of the present invention, there is provided a method for preparing the yellow azo hybrid pigment described in the first aspect. The method includes the following steps:
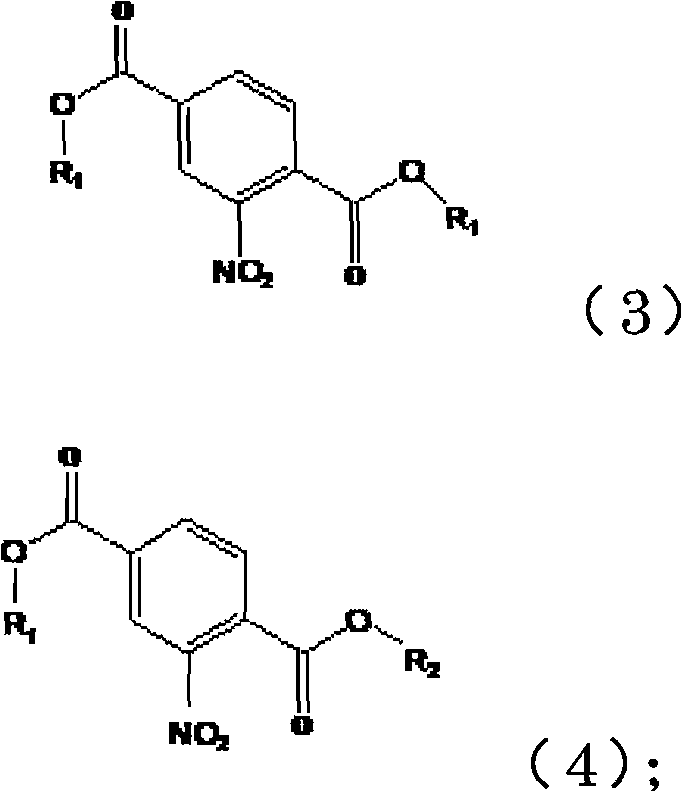
[0051] (1) Under acidic catalysis, dimethyl 2-nitroterephthalate is transesterified in alcohol to obtain 2-nitroterephthalic acid mixed esters of the following formulas (3) and (4)
[0052]
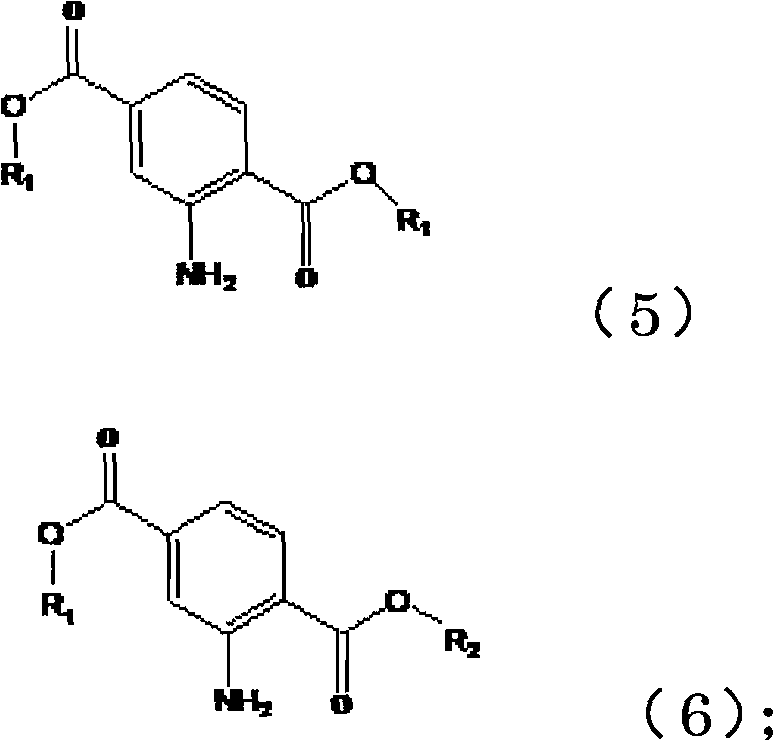
[0053] (2) Hydrogenation reduction of the above-mentioned 2-nitroterephthalic acid mixed esters to obtain the corresponding 2-aminoterephthalic acid mixed esters of the following formulas (5) and (6)
[0054]
[0055] (3) Diazotizing the above-mentioned 2-aminoterephthalic acid mixed ester at a temperature of 0-10°C;
[0056] (4) Dissolve 5-acetylacetamidobenzimidazolone as a coupling component in an aqueous solution of sodium hydroxide, add an emulsifier to obtain a coupling solution, and then add sodium bicarbonate to the bottom water of the coupling. At the temperature, the diazonium compou...
Example Embodiment
[0085] Example 1 Preparation of 2-aminoterephthalic acid mixed ester
[0086] Add 30 grams of dimethyl 2-nitroterephthalate to 300 milliliters of butanol, then add 0.1 grams of concentrated sulfuric acid and keep it under reflux for 5 hours. The resulting substance is detected by high performance liquid chromatography to contain 2-nitro-p-benzene. The mixed ester of dimethyl dicarboxylate and methyl 2-nitro-4-n-butoxycarbonyl benzoate, the content of the mixed ester is 99.3%, of which dimethyl 2-nitroterephthalate and 2-nitro The weight ratio of methyl 4-n-butoxycarbonylbenzoate is 95:5.
[0087] The temperature was lowered to normal temperature, the above reactants were added to the autoclave, 0.1 g of 3% palladium-carbon catalyst was added, and hydrogen was introduced at 110° C. and reacted at 0.2 mpa for 10 hours. The temperature is lowered to 90°C, and the palladium carbon catalyst is recovered by hot filtration. The mother liquor was collected, cooled to normal temperature,...
Example Embodiment
[0088] Example 2 Preparation of 2-aminoterephthalic acid mixed ester
[0089] Add 30 grams of dimethyl 2-nitroterephthalate to 300 ml of butanol, then add 0.15 g of concentrated sulfuric acid, and keep it under reflux for 11 hours. High performance liquid chromatography detects that it contains dimethyl 2-nitroterephthalate. The mixed ester of methyl ester and 2-nitro-4-n-butoxycarbonyl benzoate, the content of mixed ester is 99.8%, of which dimethyl 2-nitroterephthalate and 2-nitro-4- The weight ratio of methyl n-butoxycarbonyl benzoate is 85:15.
[0090] The temperature was lowered to normal temperature, the reactants were added to the autoclave, 0.1 g of 3% palladium-carbon catalyst was added, and hydrogen was introduced at 110° C. and reacted for 10 hours at 0.2 mpa. The temperature is lowered to 90°C, and the palladium carbon catalyst is recovered by hot filtration. The mother liquor was collected, cooled to normal temperature, allowed to stand for 5 hours, filtered, washed...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap