Application of furocoumarin compounds for preparing vasodilators
A furanocoumarin, vasodilation technology, applied in the directions of active ingredients of heterocyclic compounds, drug combinations, cardiovascular system diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] The present invention will be described in detail below in conjunction with the embodiments.
[0022] 1. Experimental materials
[0023] Animals: male SD rats, weighing 200-300 g, from the Experimental Animal Center of Xi'an Jiaotong University School of Medicine.
[0024] Instrument: DMT vascular tension measurement system (Danish Myo Technology AIS Inc. 610M).
[0025] Reagent: Krebs solution (composition (g / L): 6.954NaCl, 0.343KCl, 1.260NaHCO- 3 , 0.187NaH 2 PO 4 2H 2 O, 0.166CaCl 2 , 0.244MgCl 2 ·6H 2 O, 1.090 glucose, pH=7.2~7.4), dimethylsulfoxide (DMSO, SIGMA.Inc), norepinephrine (NE), U46619.
[0026] 2. Experimental method
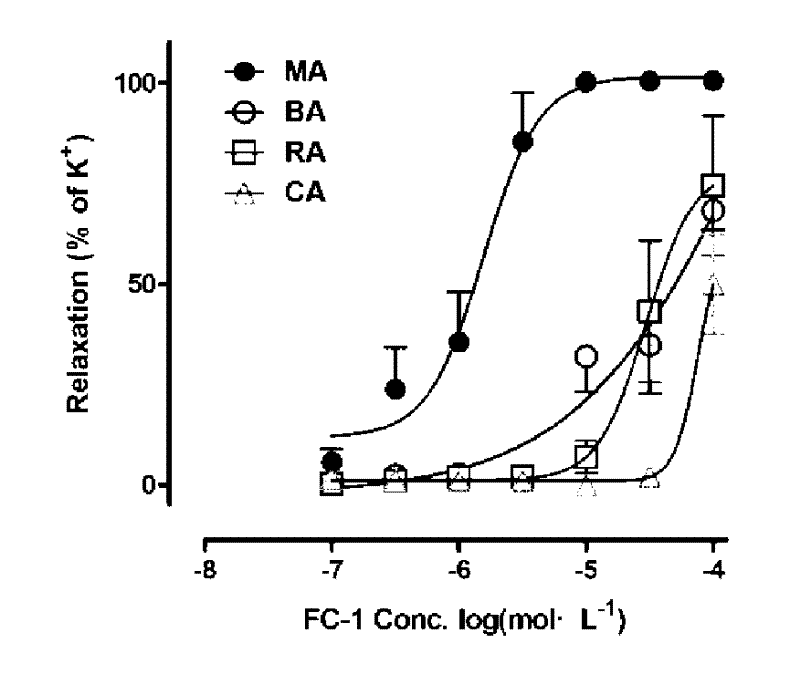
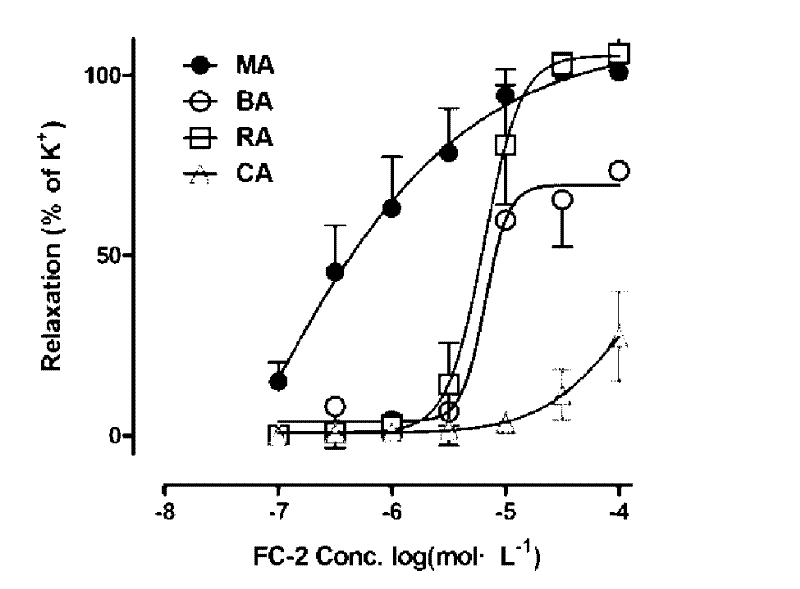
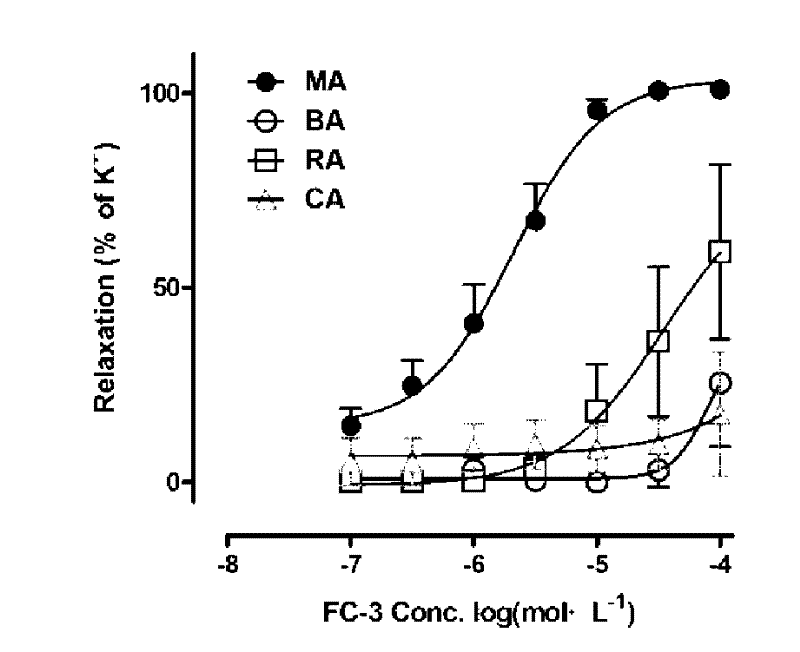
[0027] After the rats were killed by neck dislocation, the secondary branches of the mesenteric artery (MA), renal artery (RA), coronary artery (CA), and cerebral basilar artery (BA) were taken out, placed in pre-cooled Krebs solution, and made into 3 mm long branches. Vascular ring specimens are connected with the DMT vascular te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com