Modified borane ammoniate hydrogen storage material and preparation method thereof
A hydrogen storage material, borane ammonia technology, applied in chemical instruments and methods, metal hydrides, inorganic chemistry, etc., can solve the problems of generating large volatile impurity gases, restricting the application of hydrogen storage, etc., and achieve a good application prospect of hydrogen storage , Suppression of impurity gas release, high hydrogen discharge capacity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
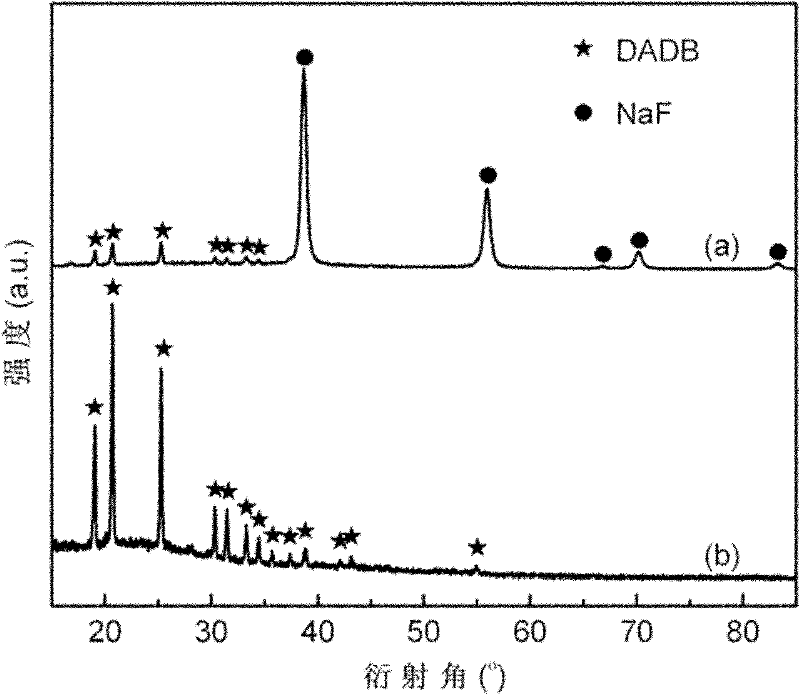
[0030] First, with NaBH 4 and NH 4 F is the starting material, the molar ratio is 1:1, and the mixture of DADB / 2NaF is prepared by mechanical ball milling method; then the by-product NaF in the ball milled sample is removed by dissolving, filtering, deammonizing and other steps with liquid ammonia as solvent, A white DADB powder was obtained as follows:
[0031] The raw material used is: NaBH 4 (purity 98%, ~200 mesh), NH 4 F (purity 98%, ~200 mesh).
[0032] NaBH in an argon atmosphere glove box 4 / NH 4 The F mixture and stainless steel balls were put into a stainless steel ball mill jar, sealed with a cover, and placed on a Fritsch 7 planetary ball mill for 3 hours of ball milling. The ball milling atmosphere is high-purity argon (purity 99.9999%), the initial pressure is 1 atmosphere, and the mass ratio of balls to materials is about 100:1. Put the ball-milled sample into a three-necked round-bottomed flask filled with liquid ammonia solvent, stir ultrasonically for ...
Embodiment 2
[0039] First, with LiBH 4 and NH 4 Cl is the starting material, the molar ratio is 1:1, the ball milling time is 1 hour, and the rest of the preparation conditions are the same as in Example 1.
[0040] The raw material used is: LiBH 4 (purity 98%, ~200 mesh), NH 4 Cl (purity 98%, ~200 mesh).
[0041] X-ray diffraction test conditions are the same as in Example 1. Figure 4 gives (a) LiBH 4 / NH 4 X-ray diffraction pattern of Cl ball-milled sample and (b) DADB after purification. The results showed that LiBH 4 / NH 4 The Cl mixture reacts completely according to the stoichiometric ratio during the ball milling process to form a mixture of DADB and LiCl. The X-ray diffraction pattern of DADB and the results reported in the literature [1.S.G Shore and K.W. Inorg.Chem.1964, 3, 914-915] consistent; the liquid ammonia purification method can effectively remove the by-product LiCl, and finally obtain a white DADB powder sample with a particle size of about 300 mesh, a purity...
Embodiment 3
[0047] First, with LiBH 4 and (NH 4 ) 2 SO 4 as the starting material, the molar ratio is 2:1, the ball milling time is 2 hours, and the rest of the preparation conditions are the same as in Example 1.
[0048] The raw material used is: LiBH 4 (purity 98%, ~200 mesh), (NH 4 ) 2 SO 4 (purity 98%, ~200 mesh).
[0049] X-ray diffraction test conditions are the same as in Example 1. Figure 7 gives (a)2LiBH 4 / (NH 4 ) 2 SO 4 The X-ray diffraction pattern of the ball-milled sample and (b) DADB after purification. The results showed that 2LiBH 4 / (NH 4 ) 2 SO 4 During the ball milling process, the mixture reacted completely according to the stoichiometric ratio to form DADB and Li 2 SO 4 The mixture, the X-ray diffraction pattern of DADB and the results reported in the literature [1.S.G Shore and K.W. Inorg.Chem.1964,3,914-915] consistent; adopting the liquid ammonia purification method can effectively remove the by-product Li 2 SO 4 , finally obtained a white ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap