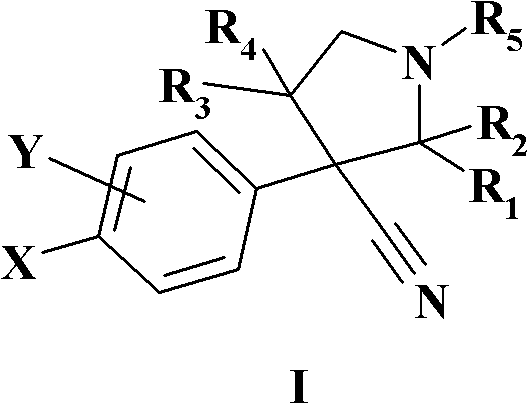
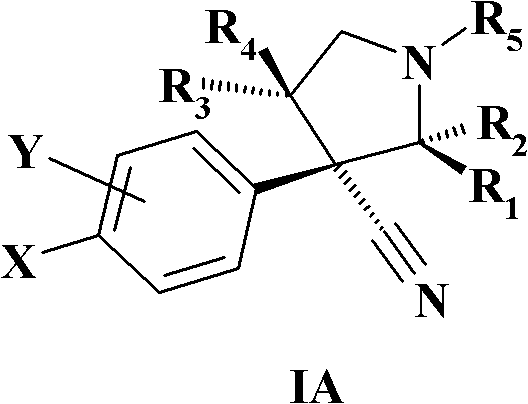
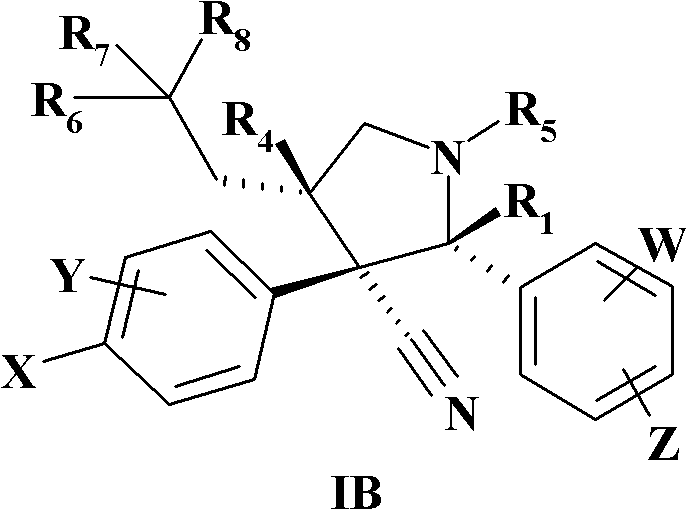
Novel N-substituted-pyrrolidines as inhibitors of MDM2-p53 interactions
A compound and alkyl technology, applied in the field of pyrrolidine-2-carboxamide derivatives I, can solve problems affecting the degradation of MDM2 protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1a
[0225] Preparation of intermediate [1-(3-chloro-2-fluoro-phenyl)-meth-(E)-ylidene]-methyl-amine
[0226]
[0227] Molecular weight 171.6 C 8 h 7 FClN
[0228] 3-Chloro-2-fluoro-benzaldehyde (Oakwood, 97%) (1.59 g, 10.0 mmol) and methylamine (2.0 M in THF (tetrahydrofuran), Aldrich, 7.5 mL, 15.0 mmol) were dissolved in CH 2 Cl 2 (20 mL) was stirred overnight at room temperature. The reaction mixture was concentrated and the residue was dried under reduced pressure to give [1-(3-chloro-2-fluoro-phenyl)-meth-(E)-ylidene]-methyl-amine (1.72 g, 100%) , which was a colorless oil which was used in the next step without further purification.
Embodiment 1b
[0230] Preparation of intermediate [1-(3-chloro-phenyl)-meth-(E)-ylidene]-methyl-amine
[0231]
[0232] Molecular weight 153.6 C 8 h 8 ClN
[0233] 3-Chloro-benzaldehyde (Aldrich, 97%) (4.21 g, 30.0 mmol) and methylamine (2.0 M in THF, Aldrich, 22.5 mL, 45.0 mmol) were dissolved in CH 2 Cl 2 (50 mL) was stirred overnight at room temperature. The reaction mixture was concentrated and the residue was dried under reduced pressure to give [1-(3-chloro-phenyl)-meth-(E)-ylidene]-methyl-amine (4.60 g, 100%) as free A colored oil was used in the next step without further purification.
Embodiment 1c
[0235] Preparation of intermediate [1-(4-chloro-phenyl)-meth-(E)-ylidene]-methyl-amine
[0236]
[0237] Molecular weight 171.6 C 8 h 7 FClN
[0238] 4-Chloro-2-fluoro-benzaldehyde (Matrix Sci, 3.17 g, 20.0 mmol) and methylamine (2.0 M in THF, Aldrich, 15 mL, 15.0 mmol) were dissolved in CH 2 Cl 2 (20 mL) was stirred overnight at room temperature. The reaction mixture was concentrated and the residue was dried under reduced pressure to give [1-(4-chloro-2-fluoro-phenyl)-meth-(E)-ylidene]-methyl-amine (3.32 g, 97.0%) , which was a colorless oil which was used in the next step without further purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com