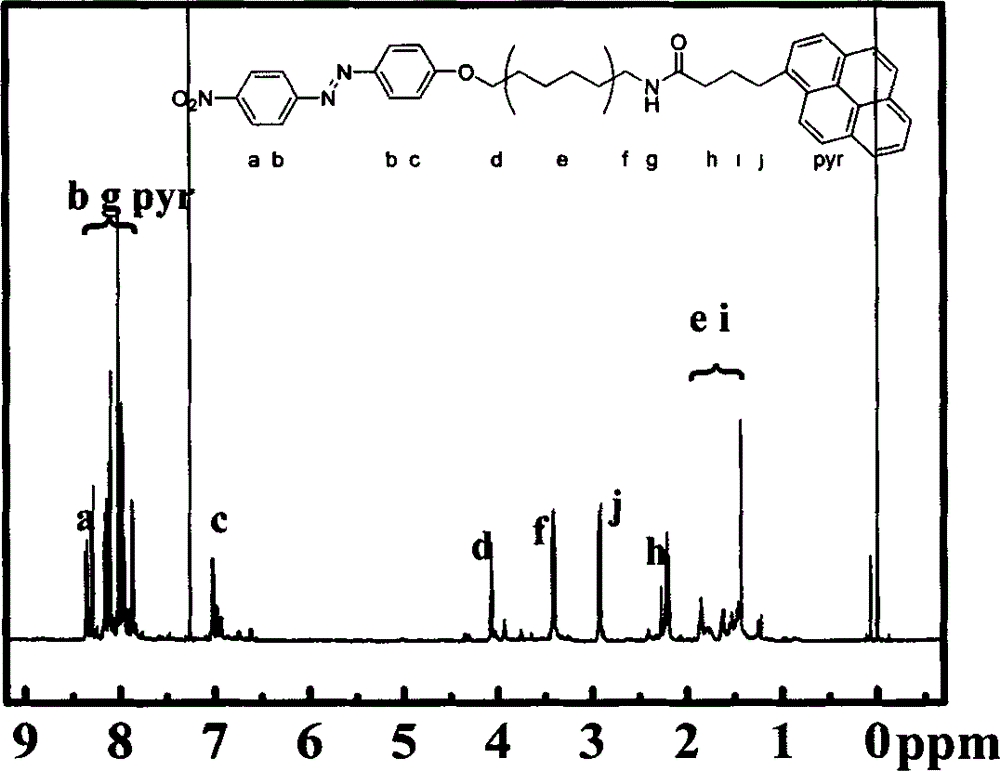
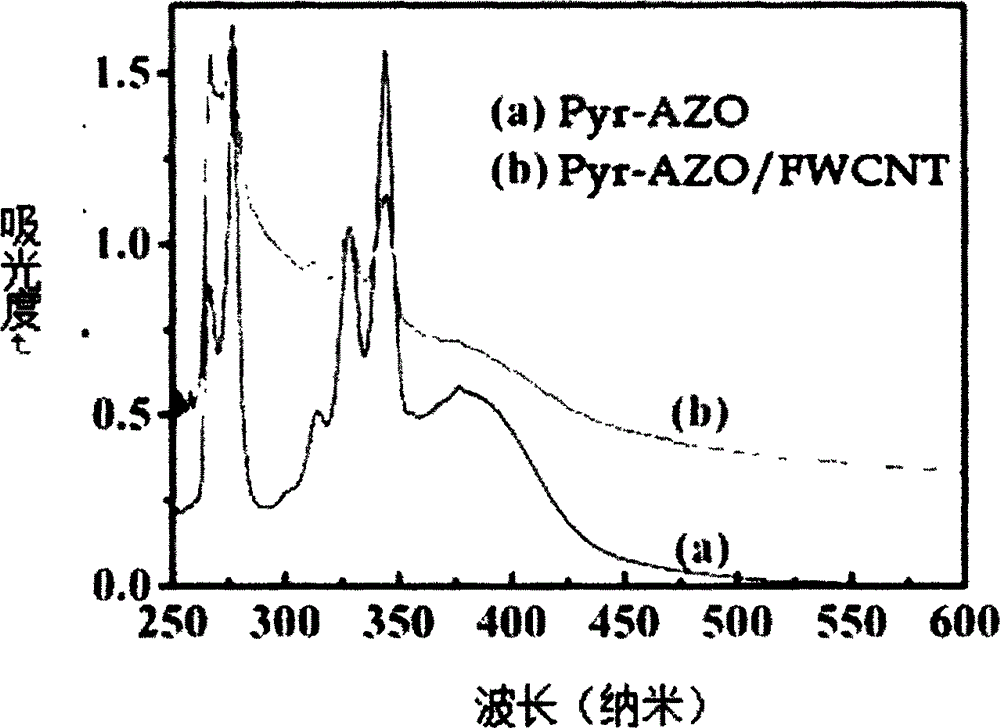
Preparation for photo-response Pyr-AZO non-covalent decorating carbon nano tube material
A non-covalent modification, carbon nanotube technology, applied in nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., to achieve good solubility, simple preparation process, and good photoresponse characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 1) Carbon nanotube treatment: add 20 mg of less-walled carbon nanotubes to 20 ml of a mixed solution with a mass fraction of 98.3% concentrated sulfuric acid and a mass fraction of 65% concentrated nitric acid in a volume ratio of 3:1, and make the mixed solution at a temperature of 70° C. Reflux for 40 minutes. After diluting the mixed acid solution with deionized water, filter and recover the filter cake, wash the filter cake with deionized water until the pH of the filtrate is 6-7, and dry in vacuum for 48 hours;
[0021]2) Synthesis of 1-amino-6-[4-(4'-nitrophenylazo)phenoxy]hexane: under stirring, 1.38g p-nitroaniline was dissolved in 20ml 3mol / L HCl aqueous solution , recorded as solution A; add 0.83g of sodium nitrite to 5ml deionized water, and record as solution B; mix solution A and solution B, and record as solution C; react in a water bath for 40 minutes. Dissolve 0.94g of phenol and 4g of NaOH in 20ml of deionized water, and record it as solution D; mix so...
Embodiment 2
[0025] 1) Carbon nanotube treatment: add 20 mg of less-walled carbon nanotubes to 20 ml of a mixed solution with a mass fraction of 98.3% concentrated sulfuric acid and a mass fraction of 65% concentrated nitric acid in a volume ratio of 3:1, and make the mixed solution at a temperature of 70° C. Reflux for 40 minutes. After diluting the mixed acid solution with deionized water, filter and recover the filter cake, wash the filter cake with deionized water until the pH of the filtrate is 6-7, and dry in vacuum for 48 hours;
[0026] 2) Synthesis of 1-amino-6-[4-(4'-nitrophenylazo)phenoxy]hexane: same as 2) in Example 1;
[0027] 3) Synthesis of pyrene-azobenzene organic molecules: Weigh 128 mg of pyrene butyric acid and dissolve it in 80 ml of anhydrous DMF, add 96 mg of N-hydroxysulfosuccinimide (Sulfo-NHS), 4-dimethylaminopyridine ( DMAP) 54 mg, add 68 mg of 1-ethyl-3-(3-trimethylaminopropyl) carbodiimide hydrochloride (EDC·HCl) under constant stirring in an ice-water bath, ...
Embodiment 3
[0031] 1) Carbon nanotube treatment: add 20 mg of less-walled carbon nanotubes to 20 ml of a mixed solution with a mass fraction of 98.3% concentrated sulfuric acid and a mass fraction of 65% concentrated nitric acid in a volume ratio of 3:1, and make the mixed solution at a temperature of 70° C. Reflux for 40 minutes. After diluting the mixed acid solution with deionized water, filter and recover the filter cake, wash the filter cake with deionized water until the pH of the filtrate is 6-7, and dry in vacuum for 48 hours;
[0032] 2) Synthesis of 1-amino-6-[4-(4'-nitrophenylazo)phenoxy]hexane: same as 2) in Example 1;
[0033] 3) Synthesis of pyrene-azobenzene organic molecules: Weigh 128 mg of pyrene butyric acid and dissolve it in 20 ml of anhydrous DMF, add 180 mg of N-hydroxysulfosuccinimide (Sulfo-NHS), 4-dimethylaminopyridine ( DMAP) 98 mg, add 102 mg of 1-ethyl-3-(3-trimethylaminopropyl) carbodiimide hydrochloride (EDC·HCl) under constant stirring in an ice-water bath...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap