Sythesis of optically active intermediate for the preparation of montelukast
A system and technology of ruthenium complexes, applied in the field of synthesis of optically active alcohols, can solve problems such as difficult preparation of ligands
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
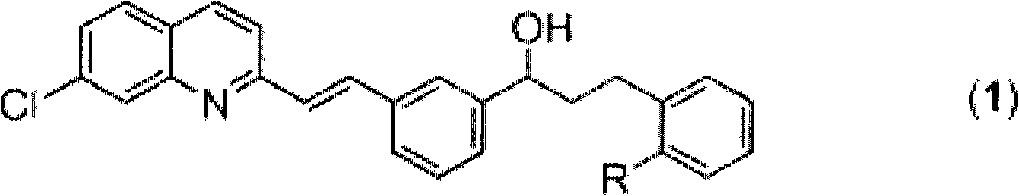
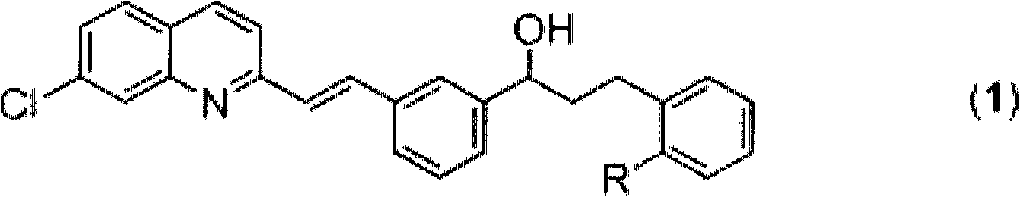
[0032] (E)-2-[3-[3-[2-(7-chloro-2-quinolinyl)vinyl]phenyl]-3-hydroxypropyl]benzoic acid methyl ester ((1), R= -C(O)OCH 3 ) Screening of catalysts in the preparation
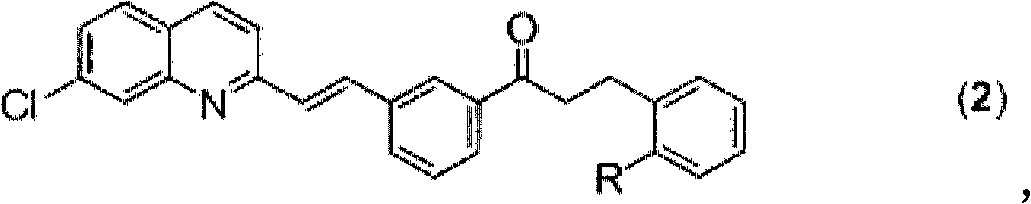
[0033] According to the ratio given in Table 1, the ketone 2 (R=-C(O)OCH 3 ), sodium formate and surfactant (methyl trioctyl ammonium chloride) are added as solids into a Schlenk tube. The vessel is placed in an inert atmosphere after three vacuum / argon cycles. Degassed water and degassed dichloromethane were added in a ratio of water: dichloromethane=1:2. The catalyst was added in the proportions given in Table 1, and the reaction mixture was heated to 40°C. Timely, the conversion rate and enantiomeric excess were monitored by HPLC analysis at different times. The results are shown in Table 1.
[0034] Table 1 Screening of transfer hydrogenation catalyst
[0035]
[0036]
Embodiment 2
[0038] (E)-2-[3-[3-[2-(7-chloro-2-quinolinyl)vinyl]phenyl]-3-hydroxypropyl]benzoic acid methyl ester ((1), R= -C(O)OCH 3 ) Screening of hydrogen donors in the preparation
[0039] According to the ratio given in Table 2 ketone 2 (R = -C (O) OCH 3 ), hydrogen donor and surfactant (methyl trioctyl ammonium chloride) as a solid added to the Hiddink tube. The container is placed in an inert atmosphere after three vacuum / argon cycles. The solvent and catalyst were added in the proportions described in Table 2, and the reaction mixture was heated to 40°C. Timely, the conversion rate and enantiomeric excess were monitored by HPLC analysis at different times. The results are shown in Table 2.
[0040] Table 2 Screening of hydrogen donors
[0041]
[0042]
[0043] a) Dichloromethane: water
[0044] b) Dichloromethane
[0045] c) Tetrahydrofuran
[0046] TEAF: Triethylamine: HCO 2 H
Embodiment 3
[0048] (E)-2-[3-[3-[2-(7-chloro-2-quinolinyl)vinyl]phenyl]-3-hydroxypropyl]benzoic acid methyl ester ((1), R= -C(O)OCH 3 ) Screening of solvents in the preparation
[0049] According to the ratio given in Table 3, ketone 2 (R=-C(O)OCH 3 ), sodium formate, methyl trioctyl ammonium chloride (surfactant) as a solid added to the Hiddink tube. The container is placed in an inert atmosphere after three vacuum / argon cycles. (Mesitylene)-Ru-Ms-DPEN as a catalyst and a solvent were added in the ratio described in Table 3, and the reaction mixture was heated as shown. Timely, the conversion rate and enantiomeric excess were monitored by HPLC analysis at different times. The results are shown in Table 3.
[0050] Table 3 Screening of solvents
[0051]
[0052] a) Dichloromethane: water
[0053] d)Chlorobenzene: water
[0054] e) Toluene: water
[0055] f) Ethyl acetate: water
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap