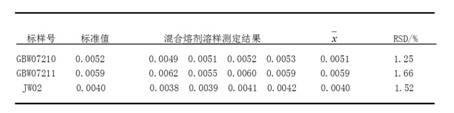
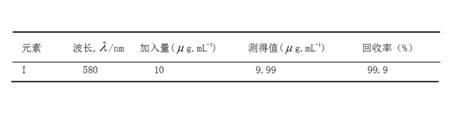
Method for detecting iodine content in phosphate ore
A technology of iodine content and phosphate rock, applied in the field of detection, can solve the problems of large influence on the environment and analysis operators, long time consumption, etc., and achieve the effects of accurate results, high accuracy and tightness of results, and time saving.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] First prepare the main reagents:
[0018] ①Sodium bicarbonate-zinc oxide mixed flux: Weigh 70g of anhydrous sodium bicarbonate and 30g of zinc oxide, put them in a mortar and grind and mix evenly. ②The volume concentration of phosphoric acid solution is: 1+1, 1+2; the volume concentration of sulfuric acid solution is: 1+9. ③ Sodium chloride saturated solution; sodium formate solution: 100 g / L, 200 g / L, 300 g / L; potassium iodide solution: 9 g / L, 10 g / L, 11 g / L; bromine water saturated solution; starch solution prepared before use: 4 g / L, 5g / L, 6g / L. ④Iodine standard solution: 100ug / mL. Weigh 0.1308g of high-grade pure potassium iodide pre-dried at 105-110°C to a constant amount, dissolve in water, transfer it to a 1000mL volumetric flask, dilute to the mark with water, and shake well. 1mL of this solution contains 100ug of iodine. ⑤ Iodine standard solution: 5ug / mL. Pipette 25.0mL of iodine standard solution into a 500mL volumetric flask, dilute to the mark with wa...
Embodiment 2
[0027] On the basis of the experimental method of Example 1,
[0028] Weigh about 0.8g of the sample to the nearest 0.0001g and place it in a porcelain crucible; add 6g of sodium bicarbonate-zinc oxide mixed flux, mix well, and then cover with 2g of mixed solvent. Put it in a high temperature furnace from low temperature to 750 °C and keep it for 40min, then take it out and cool it; pour the semi-melt in the crucible into a 100mL beaker, after leaching with water, heat it in a boiling water bath for 30min, cool it, and move it together with the precipitate into a 50mL stopper colorimetric tube, shake well. Put it into a multi-tube rack automatic balancing centrifuge, adjust the parameters at 4000 rpm, and rotate for 4 min to achieve liquid-solid separation. Draw 5.0mL of the clear solution, put it into a 25mL colorimetric tube with a stopper, neutralize it with (1+9) sulfuric acid until the Congo red test paper turns blue, and make up the volume to 8mL with water. Then opera...
Embodiment 3
[0035] On the basis of the experimental method of Example 1,
[0036] Weigh about 1 g of the sample to the nearest 0.0001 g, and place it in a porcelain crucible; add 8 g of sodium bicarbonate-zinc oxide mixed flux, mix well, and then cover with 3 g of mixed solvent. Put it in a high temperature furnace from low temperature to 800 ℃ and keep it for 50min, then take it out and cool it; pour the semi-melt in the crucible into a 100mL beaker, after leaching with water, heat it in a boiling water bath for 30min, cool it, and move it together with the precipitate into a 50mL stopper colorimetric tube, shake well. Put it into a multi-tube rack automatic balancing centrifuge, adjust the parameters at 5000 rpm, and rotate for 5 minutes to achieve liquid-solid separation. Draw 5.0mL of the clear solution, put it into a 25mL colorimetric tube with a stopper, neutralize it with (1+9) sulfuric acid until the Congo red test paper turns blue, and make up the volume to 8mL with water. Then...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap