Polyethylene glycol dog source urate oxidase analogue, and preparation method and applications thereof
A uric acid oxidase, PEGylation technology, applied in biochemical equipment and methods, oxidoreductase, enzymes and other directions, can solve the problem of failing to effectively eliminate immune response and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0040] Example 1. Modification conditions of pegylated canine urate oxidase analogs
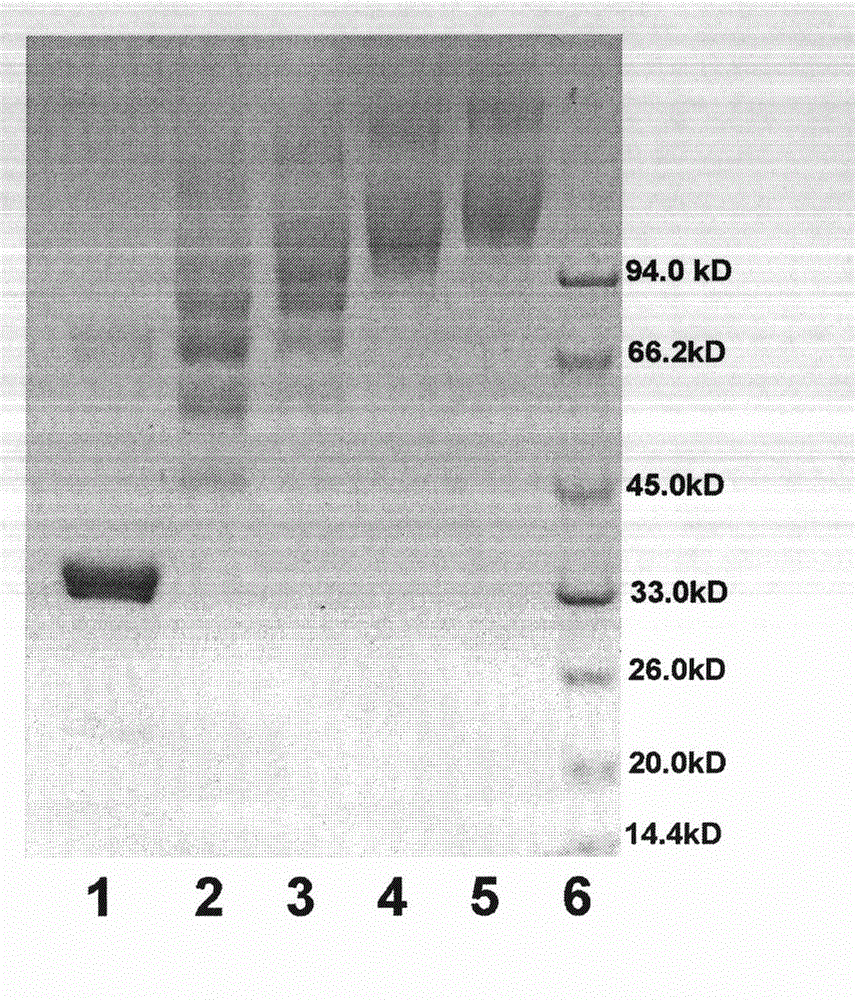
[0041] The canine urate oxidase analogue involved in this example is a mutant protein (SEQ ID NO: 5, polymorphic in the following examples) that contains a part of the canine and human urate oxidase amino acid sequences obtained by genetic engineering technology. The ethylene glycolated canine uric acid oxidase analogs all refer to the protein encoded by this sequence), and the SDS-PAGE purity and RP-HPLC purity are both higher than 95.0%.
[0042] Get Source15Q anion exchange chromatography stuffing material, pack into chromatography column, use eluent (2M NaCl 2 0.2mol / L Na 2 CO 3 -NaHCO 3 Buffer, pH=10.3) wash 5 column volumes and regenerate, use equilibrium solution (0.2mol / LNa 2 CO 3 -NaHCO 3 buffer, pH=10.3) equilibrated. Load the above-mentioned purified samples at a uniform speed, and then balance them with a balance solution until the conductance is stable. Gradient elution ...
example 2
[0046] Example 2. Separation and Purification of Pegylated Canine Urate Oxidase Analogs
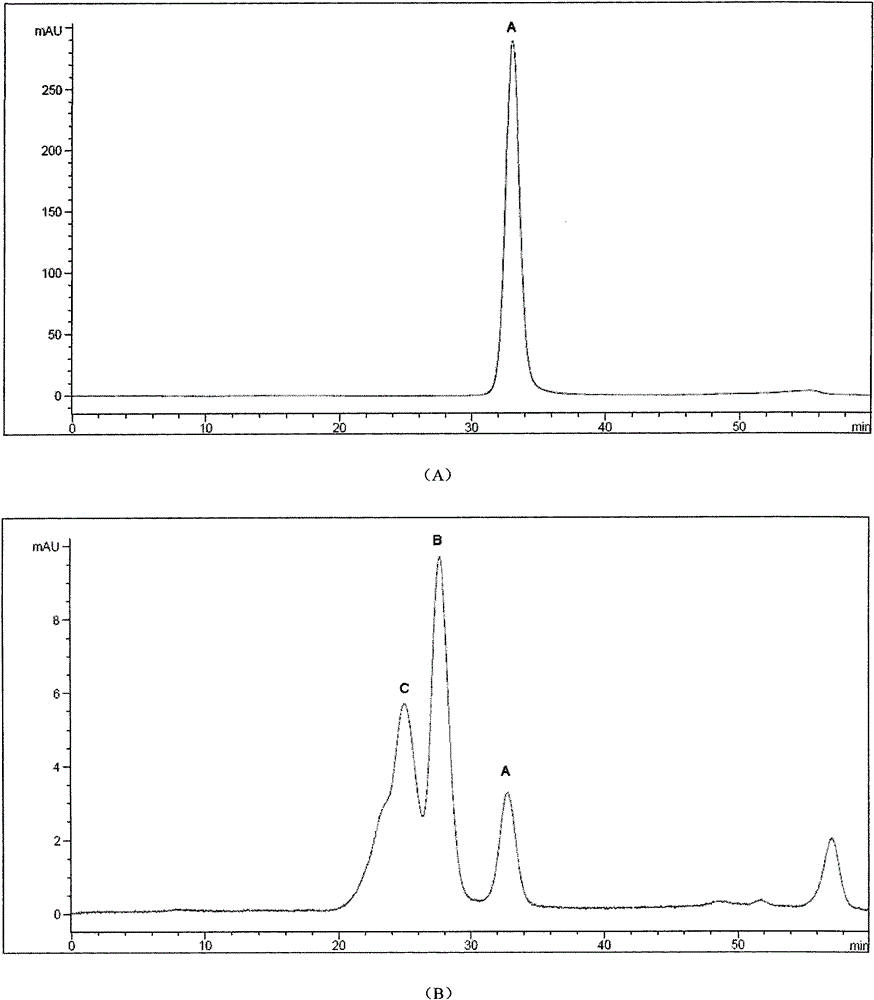
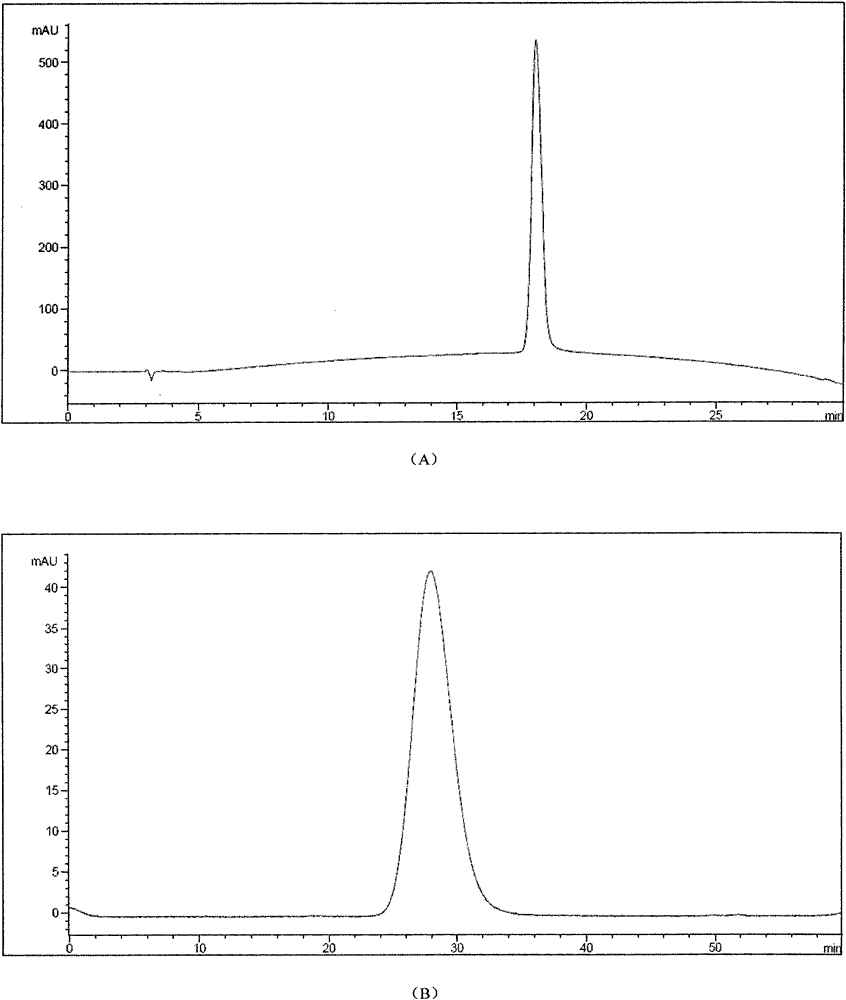
[0047] The modified canine uric acid oxidase analog sample was dialyzed overnight with 20mM PB, pH8.0. After the dialysis, the sample was concentrated by ultrafiltration (50KD Saturous tangential flow module) and then applied to a Sephacryl S300 molecular sieve chromatography column. The equilibrium buffer was 20mM PB , pH 8.0, flow rate 7.0ml / min, detect the absorbance at 254nm and 280nm at the same time after loading the sample, the first peak is the peak of the target protein, the obtained PEGylated canine urate oxidase analog protein was subjected to RP- Purity after HPLC and SEC-HPLC detection is higher than 98.0% (attached image 3 ).
example 3
[0048] Example 3. In vivo immunogenicity determination of purified proteins with different modification ratios
[0049] After four different modification ratios of 1:40, 1:80, 1:120, 1:160, the modified canine uric acid oxidase analog protein was purified according to the method described in Example 2, and then injected into mice to detect its IgG antibody titer. 24 Kunming rats were randomly divided into 4 groups (n=6), and each group was injected with canine urate oxidase analogue protein with different modified ratios, and the injection dose was 1.0 mg / kg. Inject once every five days for 6 consecutive injections. 96 hours after the last injection, 0.5 ml of blood was collected from the ophthalmic venous plexus through the capillaries, and the serum was separated by centrifugation at 3000 rpm for 15 minutes. The IgG antibody titer against the unmodified canine uric acid oxidase analog protein was detected by conventional ELISA method. The results showed that the IgG antibod...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com