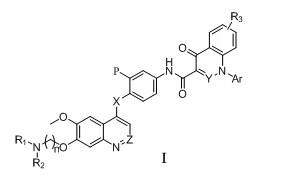
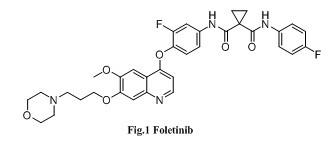
Quinoline and cinnoline compound and application thereof
一种化合物、水合物的技术,应用在含有所述化合物的药物组合物,制备治疗和/或预防癌症的药物领域,能够解决减少生物活性等问题,达到强抑制作用、抑制c-Met激酶活性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
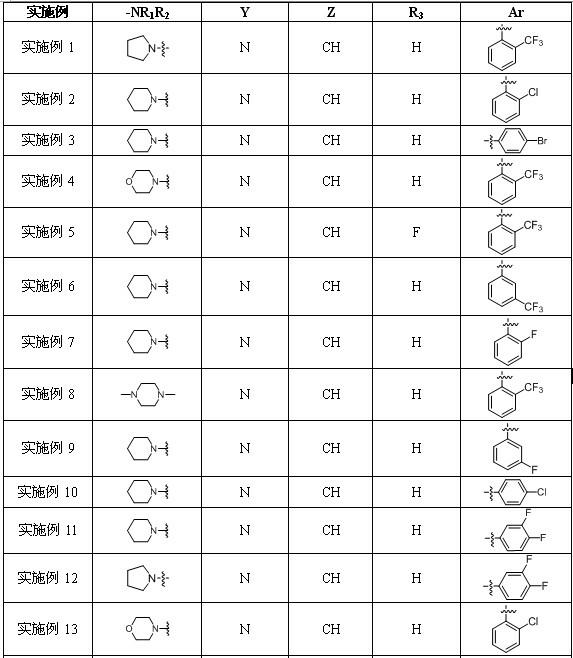
Embodiment 1
[0198] Example 1: N -[3-fluoro-4-[6-methoxy-7-[3-(1-pyrrolidinyl)propoxy)quinoline-4-oxyl)phenyl-1-(2-trifluoromethyl phenyl)-4-oxo-1,4-dihydrocinnoline-3-carboxamide dihydrochloride
[0199] Step A 1-(4-(3-chloropropoxy)-3-methoxy)acetophenone (Ⅱ)
[0200] Add 3-methoxy-4-hydroxyacetophenone (600g, 3.61moL) and anhydrous potassium carbonate (698g, 5.055moL) into DMF (5v / w, 2500mL), and stir well at 25°C for 30min , and then 1,3-bromochloropropane (795.9g, 1.4moL) was slowly added dropwise, and after the dropwise addition was completed, the reaction was stirred at 25°C for 10h. After the reaction, filter with suction, wash the filter cake with a small amount of DMF, collect the filtrate, slowly pour the filtrate into ice water and stir vigorously, a solid is precipitated, filter with suction, and dry the filter cake to obtain 827.2 g of a solid, with a yield of 93.8%.
[0201] Step B 1-(4-(3-chloropropoxy)-5-methoxy-2-nitro)acetophenone (Ⅲ)
[0202] the intermediate ...
Embodiment 2
[0233] Example 2: N -[3-fluoro-4-[6-methoxy-7-[3-(1-piperidinyl)propoxy]quinoline-4-oxyl]phenyl]-1-(2-chlorobenzene Base)-4-oxo-1,4-dihydrocinnoline-3-carboxamide dihydrochloride
[0234] ESI-MS [M+H] (m / z): 741.7; 1 H NMR (300 MHz, DMSO) δ 11.84 (s, 1H), 8.83 (d, J = 6.5 Hz, 1H), 8.40 (d, J =7.2 Hz, 1H), 8.13 (dd, J =12.8, 2.1 Hz, 1H), 7.97-7.86 (m, 3H), 7.85-7.78 (m, 3H), 7.78-7.70 (m, 3H), 7.65 (t, J =8.8 Hz, 1H), 7.10 (d, J =8.6 Hz, 1H), 7.02 (d, J = 6.4 Hz, 1H), 4.37 (t, J = 5.8 Hz, 2H), 4.07 (s, 3H), 3.50 (d, J =11.6 Hz, 4H), 2.92 (d, J = 9.6 Hz, 2H), 2.38 (d, J = 6.5 Hz, 2H), 1.81 (m, 6H).
Embodiment 3
[0235] Example 3: N -[3-fluoro-4-[6-methoxy-7-[3-(1-piperidinyl)propoxy]quinoline-4-oxyl]phenyl]-1-(4-bromobenzene base)-4-oxo-1,4-dihydrocinnoline-3-carboxamide
[0236] ESI-MS [M+H] (m / z): 752.6; 1 H NMR (300 MHz, DMSO) δ 11.78 (s, 1H), 8.55 (s, 1H), 8.51-8.38 (m, 2H), 8.00 (d, J =12.1 Hz, 1H), 7.87–7.22 (m, 10H), 6.51 (s, 1H), 4.24 (s, 2H), 3.96 (s, 3H), 3.06 (s, 6H), 2.27 (s, 2H) , 1.76 (s, 4H), 1.52 (s, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com