Method for preparing polylactic acid from lactic acid under catalysis of titanium composite catalyst
A technology of catalyst catalyzing lactic acid and composite catalyst, which is applied in the field of polymer materials, can solve the problems of limitations and few researches, and achieve the effects of short reaction time, simple production equipment and high concentration of reactants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
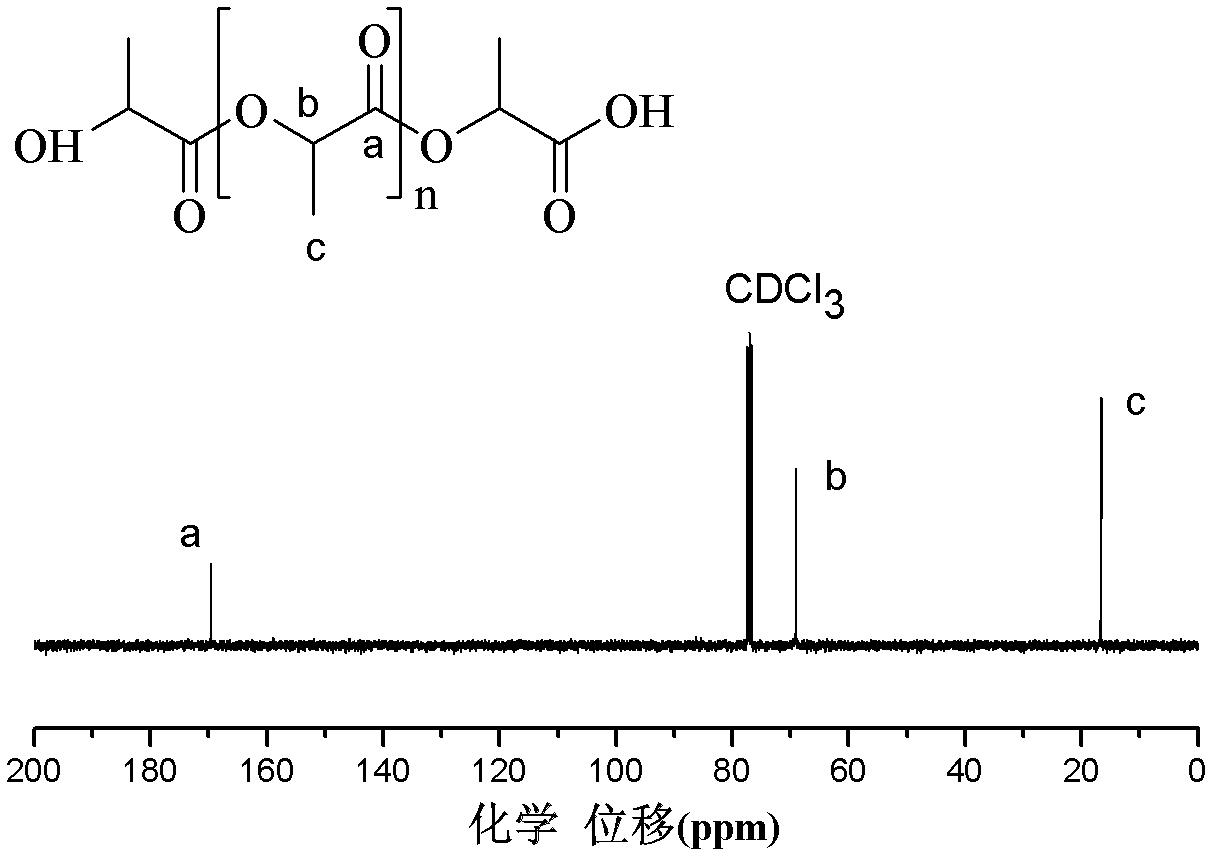
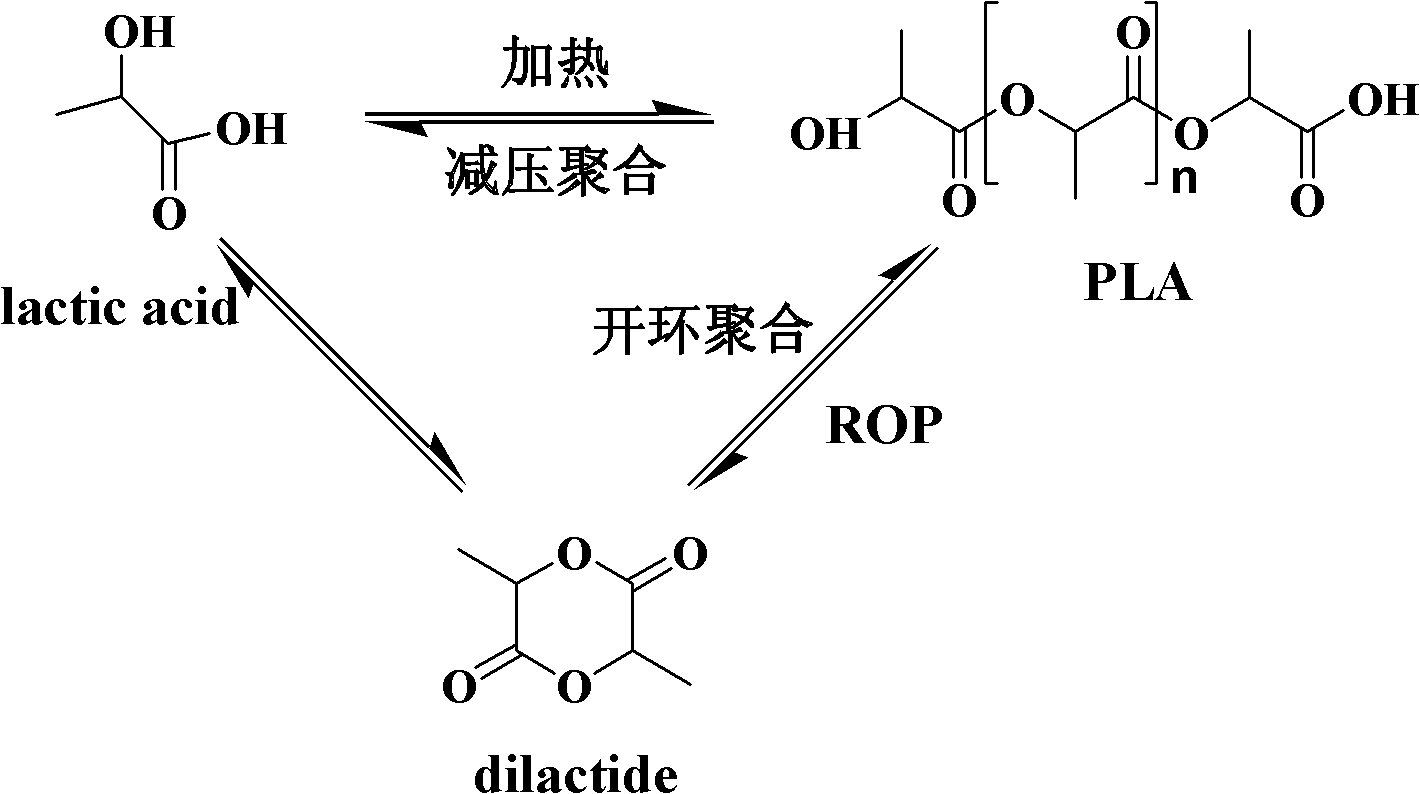
[0032] Add about 20g of lactic acid prepolymer into a 250ml three-necked flask equipped with a stirrer and a condenser tube, vacuumize and replace nitrogen for 3 to 4 times, and add tetraisopropoxytitanium and p-toluenesulfonate under the protection of nitrogen A mixture catalyst of acid (the input amount of tetraisopropoxytitanium is 0.4% of the mass of lactic acid prepolymer, and the molar ratio of tetraisopropoxytitanium to p-toluenesulfonic acid is 1:4), put the system into In an oil bath at 100°C, the system is stirred with a German IKA EUROSTAR power control visc stirrer. After the catalyst in the system is fully dissolved, the temperature is rapidly raised to 180°C and the decompression reaction is started at the same time. The vacuum degree is controlled at 30-80Pa, and the stirring speed is controlled at 400~450r / min, the polymerization reaction stops after about 25h, the product is dissolved in chloroform, precipitated in anhydrous methanol, centrifuged, the supernata...
Embodiment 2
[0034] Add about 20g of lactic acid prepolymer into a 250ml three-necked flask equipped with a stirrer and a condenser tube, vacuumize and replace nitrogen for 3 to 4 times, and add titanium tetrachloride and p-toluenesulfonic acid under the protection of nitrogen. Mixture catalyst (the input amount of titanium tetrachloride is 0.4% of the mass of lactic acid prepolymer, the molar ratio of titanium tetrachloride and p-toluenesulfonic acid is 1:4), put the system into an oil bath at 100°C , the system is stirred with a German IKA EUROSTAR power control visc stirrer. After the catalyst in the system is fully dissolved, the temperature is rapidly raised to 180°C and the decompression reaction is started at the same time. The vacuum degree is controlled at 30-80Pa, and the stirring speed is controlled at 400-450r / min. The polymerization reaction stopped after about 25 hours. The product was dissolved in chloroform, precipitated in anhydrous methanol, centrifuged, the supernatant wa...
Embodiment 3
[0036] Add about 20g of lactic acid prepolymer into a 250ml three-necked flask equipped with a stirrer and a condenser tube, vacuumize and replace nitrogen for 3 to 4 times, and add tetraisopropoxytitanium and p-toluenesulfonate under the protection of nitrogen A mixture catalyst of acid (the input amount of titanium tetraisopropoxide is 0.4% of the mass of lactic acid prepolymer, and the molar ratio of titanium tetraisopropoxide and p-toluenesulfonic acid is 1:1), put the system into In an oil bath at 100°C, the system is stirred with a German IKA EUROSTAR power control visc stirrer. After the catalyst in the system is fully dissolved, the temperature is rapidly raised to 180°C and the decompression reaction is started at the same time. The vacuum degree is controlled at 30-80Pa, and the stirring speed is controlled at 400~450r / min, the polymerization reaction stops after about 25h, the product is dissolved in chloroform, precipitated in anhydrous methanol, centrifuged, the su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com