Pyridine-containing TTF (tetrathiafulvalene) derivative and preparation method thereof
A compound and organic solvent technology, applied in the field of TTF derivatives and its preparation, can solve problems that need further research by scientists
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
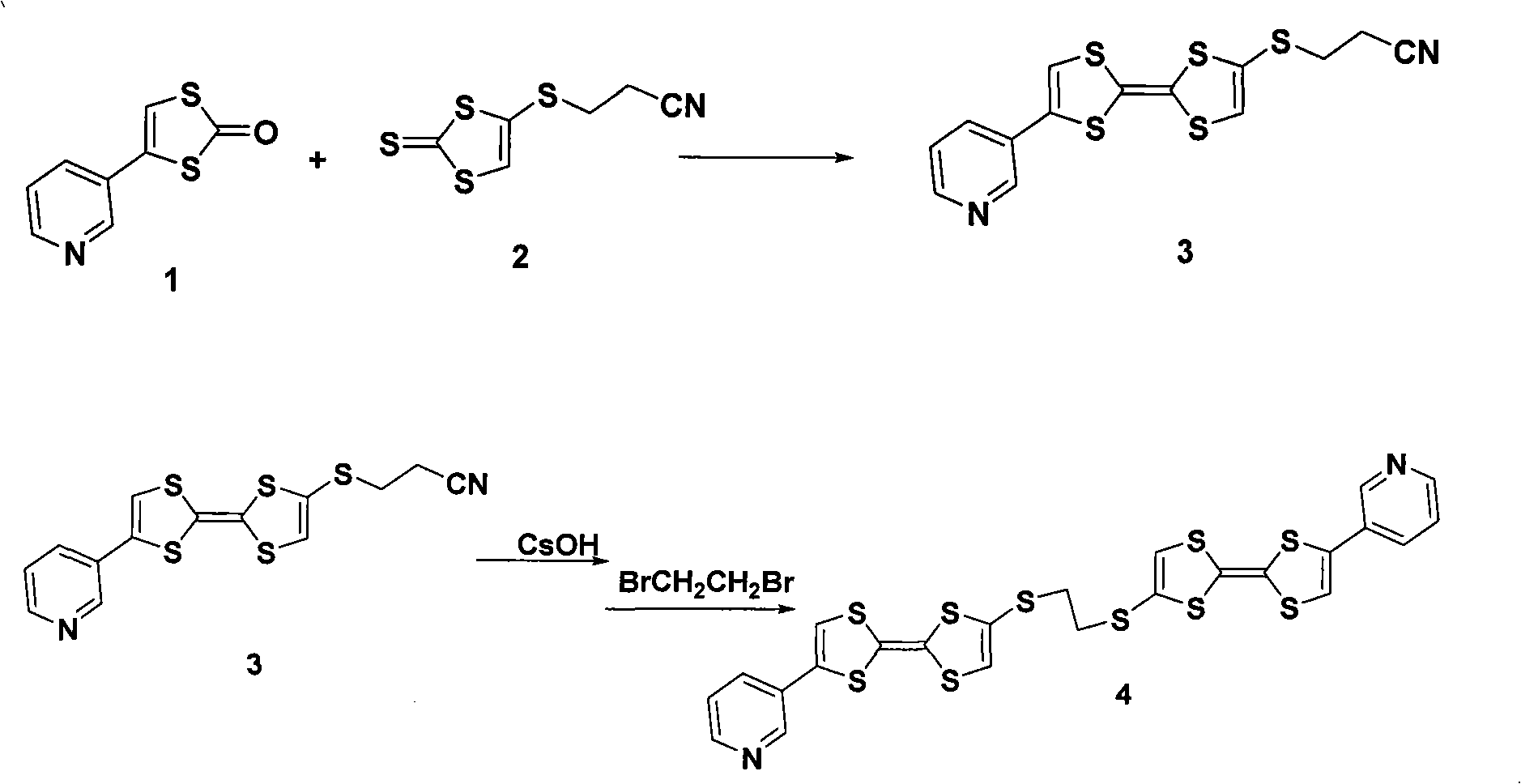
[0017] Under the protection of nitrogen, compound 1 (0.39g, 2mmol) and compound 2 (0.44g, 2mmol) were successively added into the three-necked flask, and P(OCH 3 ) 3 (10ml), stirred at 60°C for 3 hours, cooled to room temperature, and distilled off P(OCH 3 ) 3 , using silica G254 (200-300 mesh) as the filler and dichloromethane as the eluent to obtain compound 3 (0.39 g, yield 54%).
[0018] Melting point: 120-121°C.
[0019] 1 HNMR (CDCl 3 500MHZ)3.12(2H), 3.34(2H), 5.51(1H), 6.38(1H), 7.62(1H), 7.72(1H), 8.47(1H), 8,58(1H).
[0020] 13 CNMR (CDCl 3 50MHZ) 52.1 109.1 117.2 123.3 124.7 129.3 132.6 134.1 139.0 149.5 150.3
[0021] MS(EI)366
Embodiment 2
[0023] Under nitrogen protection, compound 1 (0.39g, 2mmol) and compound 2 (0.44g, 2mmol) were sequentially added to a three-necked flask, and P(OC2H5)3 (10ml) was added in one go, stirred at 60°C for 3 hours, cooled to room temperature, distilled off P(OC 2 h 5 ) 3 , using silica G254 (200-300 mesh) as a filler for column chromatography and dichloromethane as an eluent to obtain compound 3 (0.32 g, yield 45%).
Embodiment 3
[0025] Under the protection of nitrogen, compound 1 (0.39g, 2mmol) and compound 2 (0.44g, 2mmol) were successively added into the three-necked flask, and P(OCH 3 ) 3 (10ml), stirred at 120°C for 3 hours, cooled to room temperature, and distilled off P(OCH 3 ) 3 , using silica G254 (200-300 mesh) as the filler and dichloromethane as the eluent to obtain compound 3 (0.29 g, yield 40%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com