Method for the preparation of 2,5-furandicarboxylic acid and for the preparation of the dialkyl ester of 2,5-furandicarboxylic acid
A technology of furandicarboxylic acid and dialkyl esters, which is applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., and can solve problems such as not being able to obtain FDCA
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
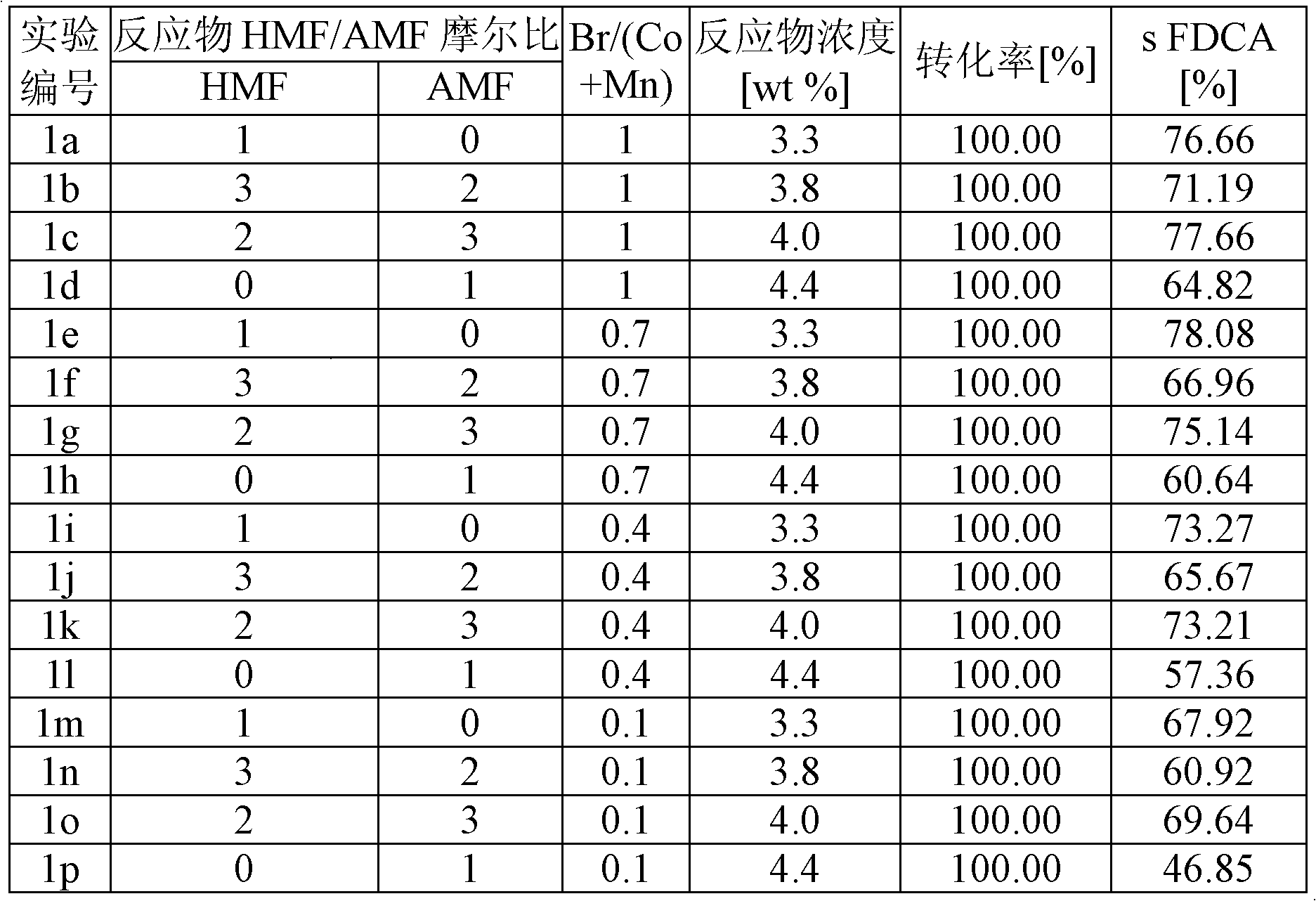
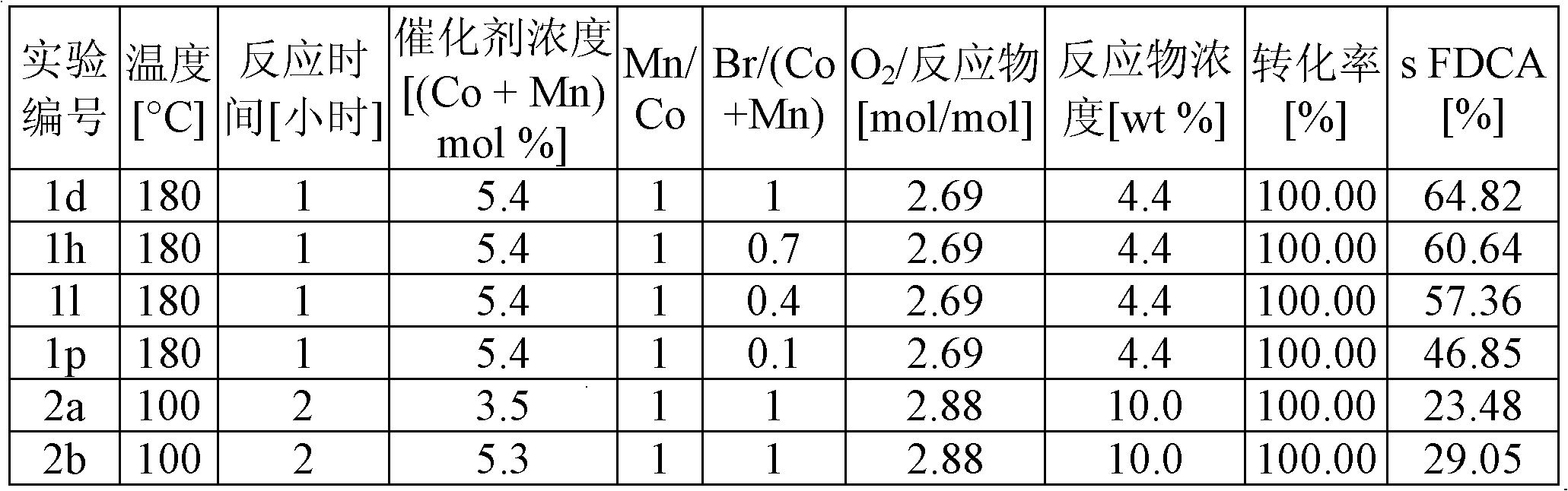
[0039] Example 1 shows the selectivity of FDCA in the oxidation of HMF, a 3 / 2 mixture of HMF / AMF, a 2 / 3 mixture of HMF / AMF, and AMF, respectively, with 2.7 mol% cobalt catalyst (relative to reactants) , and the cobalt / manganese molar ratio is 1 / 1, so that the total catalyst concentration (Co+Mn) is 5.4 mol%. The molar ratios of Br / (Co+Mn) were 1.0, 0.7, 0.4 and 0.1, the concentration of reactants in acetic acid was 0.26M, and the reaction was carried out at 180° C. and 20 bar air pressure for 1 hour. The oxygen content was 2.69 mol oxygen / mol reactant. Under these conditions, the higher the bromine content, the higher the yield, but when Br / (Co+Mn) > 1, there will be corrosion problems on an industrial scale. HMF gave slightly higher yields than AMF at one hour reaction time. The results of these experiments are listed in Table 1.
Embodiment 2
[0041] Example 2 shows the selectivity of the oxidation of AMF to FDCA of Example 1 and a comparative example based on the experimental conditions described in US 2009 / 0156841. In those comparative examples (2a and 2b), 10wt / wt% AMF in acetic acid was oxidized at 100°C and 30bar pressure for 2 hours with 1.75 and 2.65mol% cobalt catalysts and Br / (Co The molar ratio of +Mn) was fixed at 1.0, and the Co / Mn molar ratio was 1.0. The oxygen content was 2.88 mol oxygen / mol reactant. Under these conditions, the yield of FDCA was lower than the results presented in US 2009 / 0156841 and also lower than those obtained at higher temperatures. The results of these experiments are listed in Table 2.
Embodiment 3
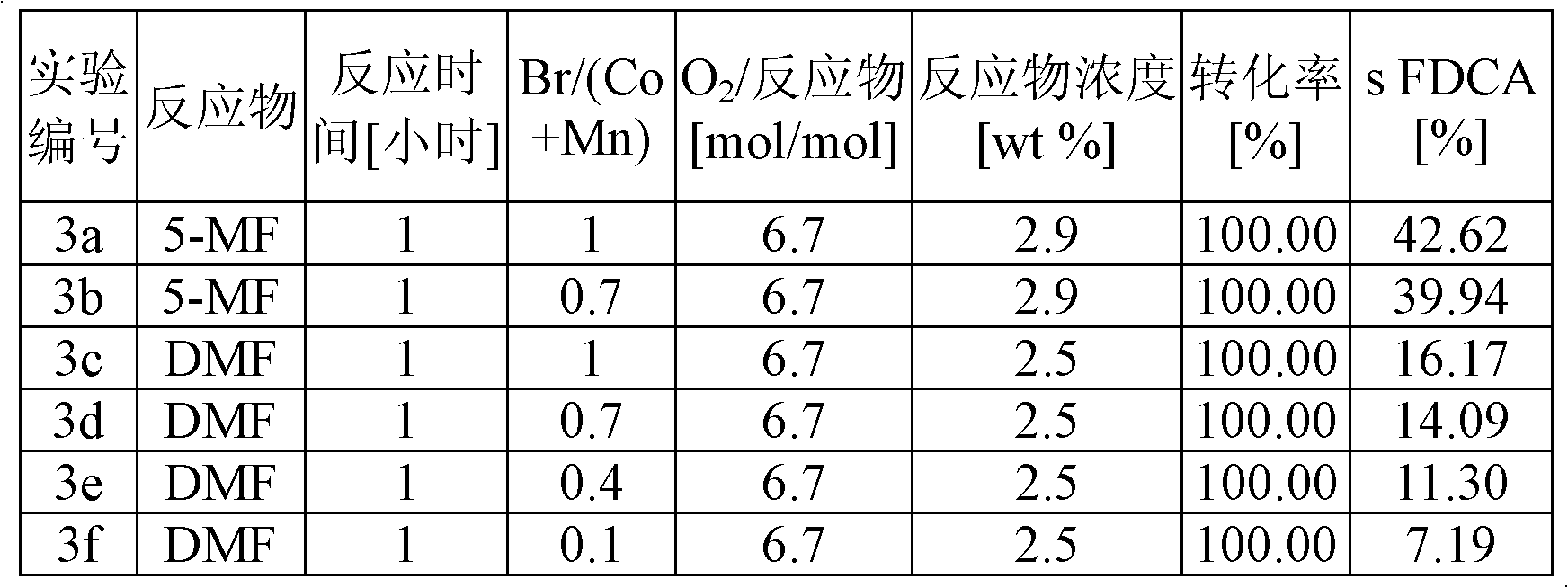
[0043] Example 3 shows the yield of FDCA in the oxidation of 5-methylfurfural (5MF) and 2,5-dimethylfurfural (DMF) at 180°C with 2.7 mol% cobalt catalyst (relative to reactants) , and the cobalt / manganese molar ratio is 1 / 1, so that the total catalyst concentration (Co+Mn) is 5.4 mol%. The molar ratios of Br / (Co+Mn) were 1.0, 0.7, 0.4 and 0.1. The concentration of the reactants in acetic acid was 0.26M. The reaction temperature was 180°C, and the reaction was carried out under an air pressure of 50 bar. The oxygen content was 6.7 mol oxygen / mol reactant. Under these conditions, the higher the bromine content, the higher the yield, but when Br / (Co+Mn) > 1, there will be corrosion problems on an industrial scale. The reaction with 5-MF gave higher yields than the reaction with DMF. The results of these experiments are listed in Table 3.
[0044] Table 1
[0045]
[0046] Table 2
[0047]
[0048] table 3
[0049]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com