Preparation method of manganese sulfate and manganese sulfate product prepared by same
A manganese sulfate and product technology, applied in the direction of manganese sulfate, etc., can solve the problems of complicated process and long process flow, and achieve the effect of simplifying the preparation process, high product purity and less impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
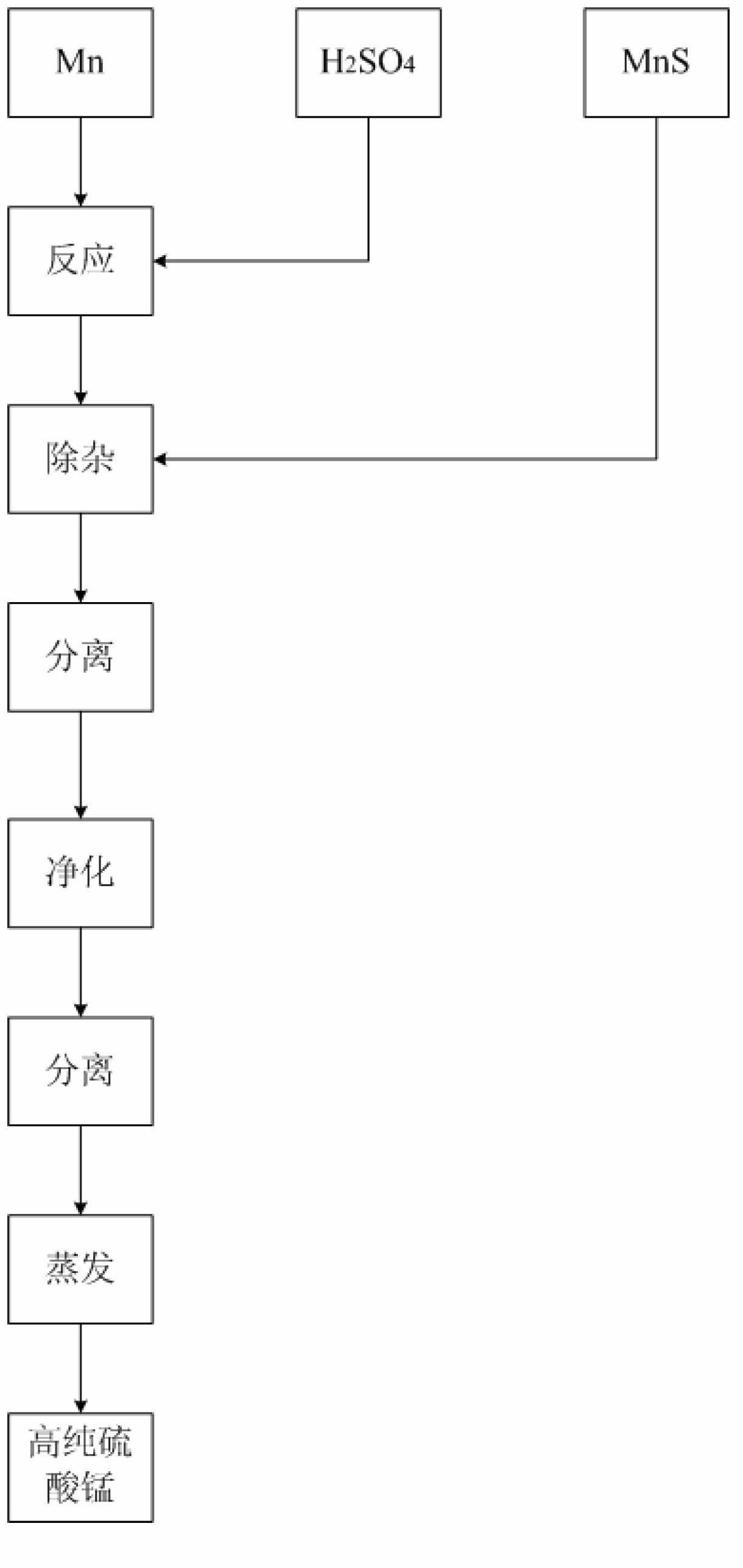
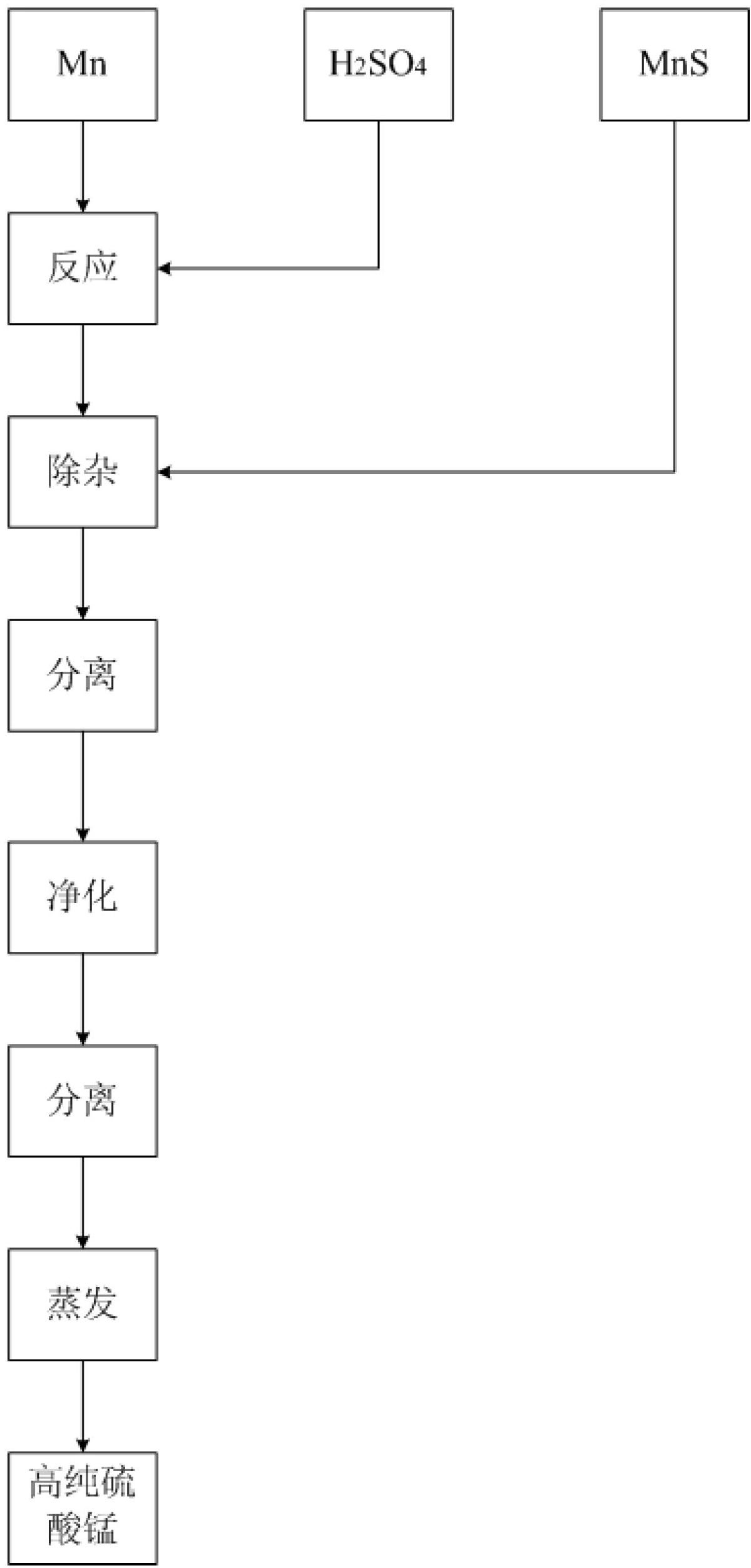
[0053] First add 4000 liters of deionized water into a 5000 liters open glass-lined reaction pot, add 500 kg of manganese metal with a purity of 99% under stirring, slowly add 98% industrial sulfuric acid to react to a pH value of 3.5, and measure the lead content to be 14.76 kg. Add 40 kg of 60% MnS, stir and react at 87°C for 5 hours, press filter to separate, recover valuable elements from the filter cake, and clarify the filtrate to proceed to the next step.
[0054] Add 27.5% industrial hydrogen peroxide to the above filtrate at a rate of 2.5L per cubic meter, stir for 15 minutes, heat to boiling, and maintain slight boiling for 30 minutes, stop heating, separate solid and liquid, discard the filter residue, and evaporate the clarified filtrate to obtain high-purity Manganese sulfate sample 1 # .
Embodiment 2
[0056] First add 4000 liters of deionized water to a 5000 liter open glass-lined reaction pot, add 600 kg of manganese metal with a purity of 99% under stirring, slowly add 98% industrial sulfuric acid to react to a pH value of 4.0, and measure the lead content to be 17.20 kg. Add 40 kg of 60% MnS, stir and react at 85°C for 5.5 hours, press filter to separate, recover valuable elements from the filter cake, and clarify the filtrate to proceed to the next step.
[0057] Add 27.5% industrial hydrogen peroxide to the above filtrate at a rate of 2.5L per cubic meter, stir for 15 minutes, heat to boiling, and maintain slight boiling for 30 minutes, stop heating, separate solid and liquid, discard the filter residue, and evaporate the clarified filtrate to obtain high-purity Manganese sulfate sample 2 # .
Embodiment 3
[0059] First add 4000 liters of deionized water into a 5000 liter open glass-lined reaction pot, add 650 kg of manganese metal with a purity of 99% under stirring, slowly add 98% industrial sulfuric acid to react to a pH value of 3.5, and measure the lead content to be 18.59 kg. Add 40 kg of 60% MnS, stir and react at 87°C for 5 hours, press filter to separate, recover valuable elements from the filter cake, and clarify the filtrate to proceed to the next step.
[0060] Add 27.5% industrial hydrogen peroxide to the above filtrate at a rate of 2.5L per cubic meter, stir for 15 minutes, heat to boiling, and maintain slight boiling for 30 minutes, stop heating, separate solid and liquid, discard the filter residue, and evaporate the clarified filtrate to obtain high-purity Manganese sulfate sample 3 # .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com