Mouse DCF1 specific polyclonal antibody and preparation method of mouse DCF1 specific polyclonal antibody
A polyclonal antibody and specific technology, which is applied in the field of mouse DCF1-specific polyclonal antibody and its preparation, can solve the problems of poor recognition effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Construction and identification of pGEX-4T1-59a and pET-30a-59c prokaryotic expression vectors
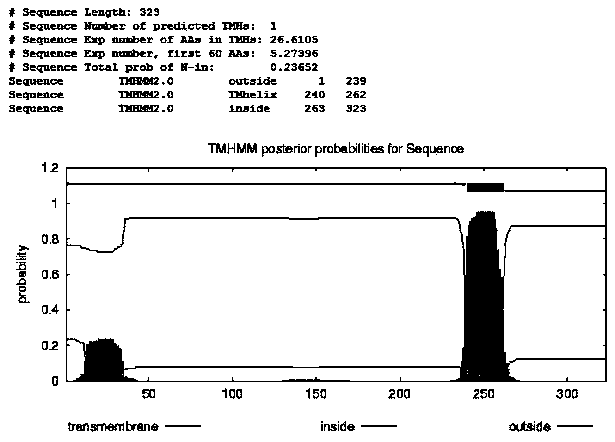
[0027] Using TMHMM Server v.2.0 to predict the protein transmembrane structure of DCF1 amino acid sequence, using Premier 5.0 software and the principle of no frameshift mutation in the expressed fusion protein to design the upstream of DCF1 extramembrane antigen sequence and Elisa substrate protein sequence and downstream primers, introducing B in its upstream and downstream primers, respectively wxya I and S al The enzyme cutting site of I and the corresponding protective bases, and the start codon and stop codon were added to the upstream and downstream primers respectively, and the primers were synthesized by Shanghai Yingwei Jieji Company. The upstream primer of the antigen sequence is: 5'-CGC GGATCC atgGACACAGCGTCCTGTCAC-3', the downstream primer is: 5'-CAAGAC GTC GAC ctaCAGCCTGCAGCCCTCTCTGG-3'; the upstream primer of the protein sequence for the Eli...
Embodiment 2
[0028] Example 2: Soluble Expression and Purification of Antigen Polypeptide Fusion Protein
[0029]Heat-shock the pGEX-4T1-59a vector into BL21(DE3) competent medium. After colonies grow on the plate, use a sterilized toothpick to pick a single colony into a test tube containing 5mL LB medium, shake at 37°C and 220rpm Cultivate overnight, add 1% of the inoculum, that is, 2mL of bacterial solution, into a conical flask containing 200mL of LB medium, and shake the OD at 37°C 600 When it was 0.8, IPTG was added to a final concentration of 0.5mM for induction culture, and after shaking culture at 37°C for 4h, centrifuge at 6000rpm for 5min to collect the bacteria into a 50 mL centrifuge tube. Place the 50 mL centrifuge tube with collected cells on ice, add 10 mL of pre-cooled cell sonication buffer, 300W, 30s sonication, 15s pause, and last for 30min. After complete crushing, put the centrifuge tube into a centrifuge, centrifuge at 11,000 rpm for 15 minutes at 4°C, collect the...
Embodiment 3
[0031] Example 3: Expression and purification of Elisa substrate protein
[0032] Heat-shock transformation of the pET-30a-59c vector into BL21(DE3) competent cells, subsequent expanded culture, induced fermentation and sonication were the same as in Example 2. Take a small amount of supernatant and precipitate respectively as samples for SDS-PAGE fusion protein soluble expression analysis, see Figure 5 -A. The results showed that the target protein was present in the supernatant phase.
[0033] Use the His tag to purify the target protein in the supernatant. For the specific process, refer to the Ni Sepharose 6 Fast Flow product manual of Amersham Biosciences, and take the components in the purification process for SDS-PAGE analysis, see Figure 5 -B. The results showed that the target protein was mainly present in the eluate, the purification was successful, and it could be used as the substrate coating protein for subsequent Elisa detection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com