Oxazolidone ring contaning adducts
A technology containing oxazolidone ring and oxazolidinone ring, which is used in epoxy resin coatings, powder coatings, organic chemistry and other directions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
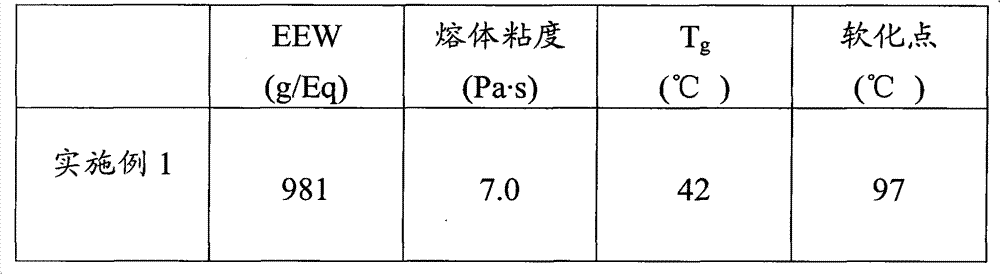
Embodiment 1
[0053] Embodiment 1 (adduct containing oxazolidinone ring)
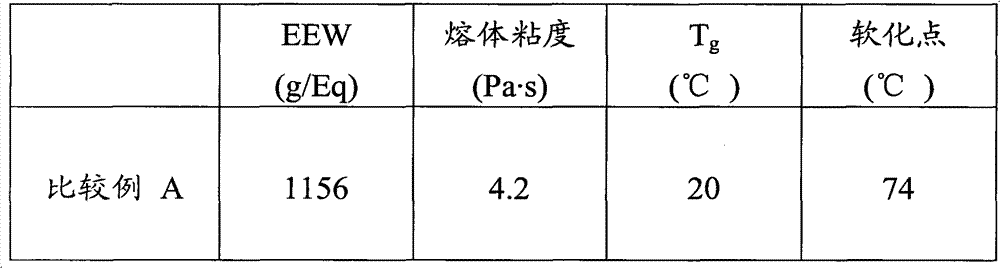
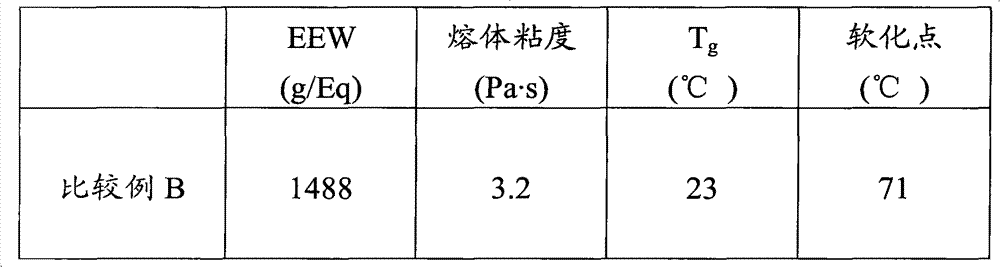
[0054] 390 grams (g) D.E.R. TM 736 was added to the flask and the contents of the flask were heated to 130°C. 2 g of DBU was added to the flask over a period of approximately 60 seconds and the contents of the flask were maintained at 145°C. 210 g of MDI was added to the flask over 60 min and the temperature of the contents of the flask was maintained at a temperature of 160°C to 170°C. The contents of the flask were kept at a temperature of 165 °C for 15 min. A sample was obtained and the EEW was determined to be about 1450. 280g D.E.R.TM 330 was added to the flask and the contents of the flask were maintained at a temperature of 140°C to 150°C. 120 g of MDI was added to the flask over 60 min and the temperature of the contents of the flask was maintained at a temperature of 170°C to 175°C. The contents of the flask were kept at a temperature of 165 °C for 15 min to generate Example 1. Epoxy equivalent weig...
PUM
| Property | Measurement | Unit |
|---|---|---|
| softening point | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com