Method for preparing a masterbatch of natural rubber and silica
A technology of silica and natural rubber, used in transportation and packaging, special tires, tire parts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
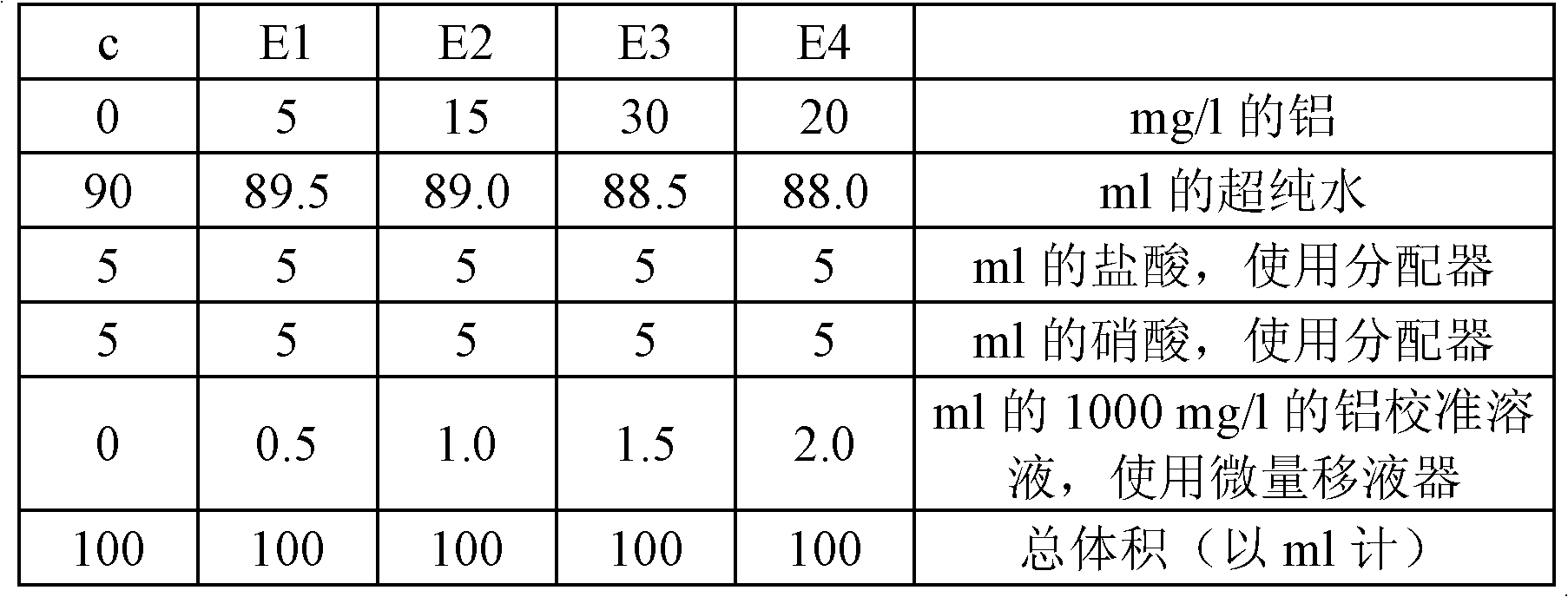
[0089] Preparation of 10 mg / l test control substance
[0090] A check control was prepared during each set of measurements in the same way as the above calibration standard by introducing different dosed amounts of 1 ml of a 1 g / l aluminum standard solution, thus enabling checking of the calibration. Test controls were not stored after use.
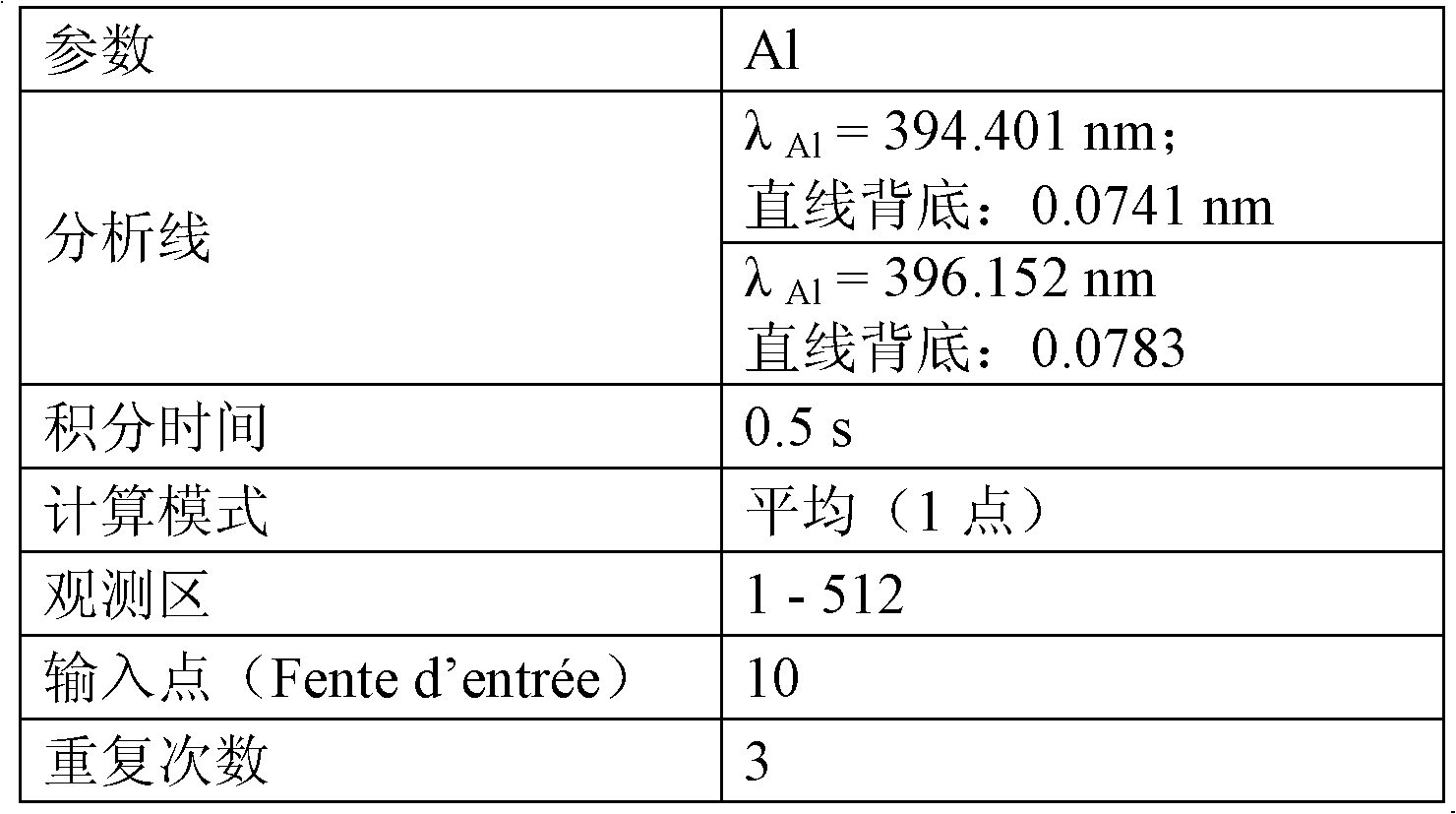
[0091] d)-4- Analysis by ICP-AES
[0092] Analysis order
[0093] 1 - calibration;
[0094] 2-10mg / l (magnesium) or 10mg / l (aluminum) test control substance;
[0095] 3-sample + blank test;
[0096] 4-E5 (20mg / l aluminum) test standard sample.
[0097] Calibration checks for the range 0-50mg / l aluminum:
[0098] Test control substance (theoretical value: 20mg / l)
[0099] Tolerance: 19.6mg / l<[Aluminum]<20.4mg / l.
[0100] Verification of the sequence of analysis in the range 0-50 mg / l aluminum (at the end of the measurement, to demonstrate the absence of drift):
[0101] E5 test standard sample (theoretical value: 50mg / l)
[0102]...
Embodiment 1
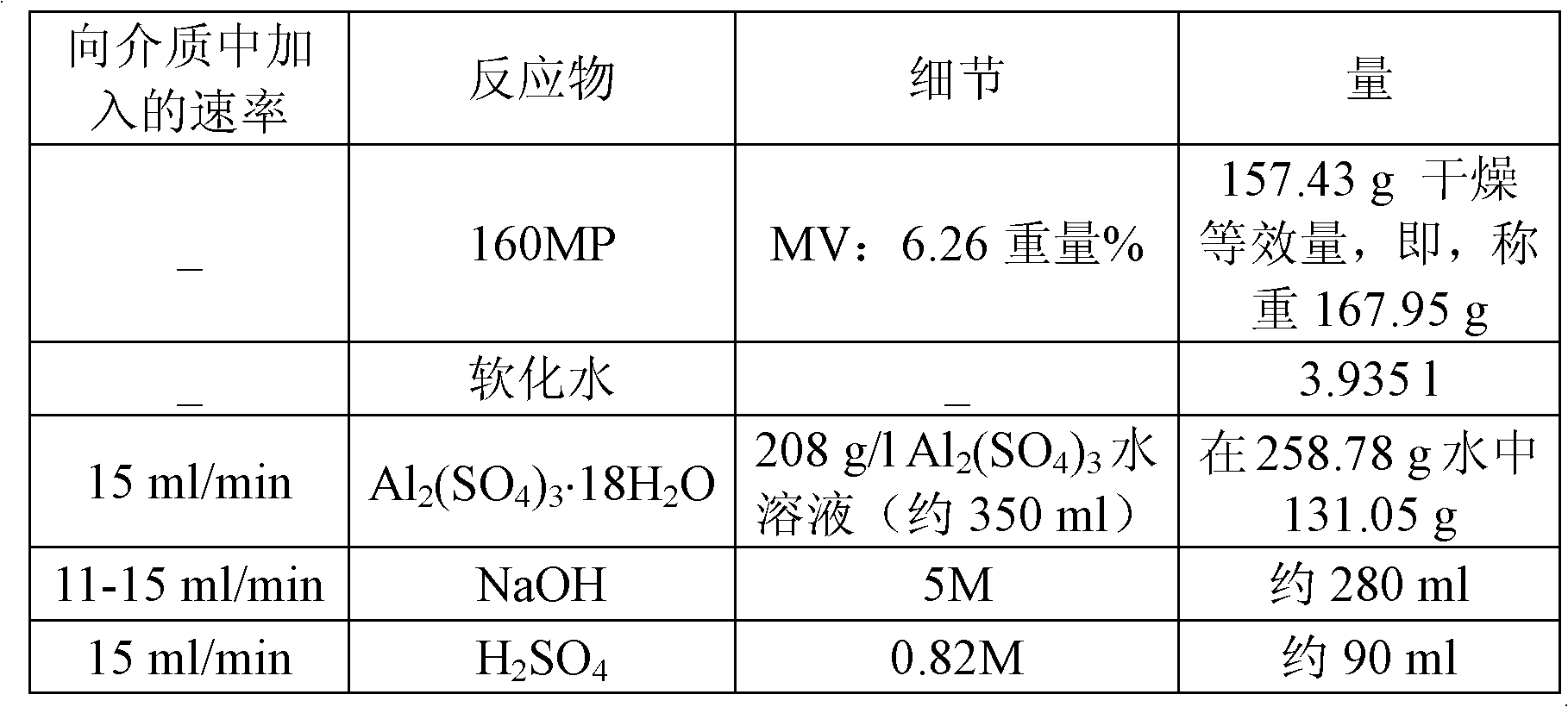
[0303] The purpose of this example is to demonstrate the proper operation of the method according to the invention, in particular the pH of the formulation measured for a given level of silica doping.
[0304] Tests E1, E2, E3 and E4 were carried out according to the method detailed in the previous section, in which:
[0305] - Natural rubber field latex from Société Africaine des Plantations d'Hévéas (SAPH; African Hevea Plantation Company) having 0.71% by weight of ammonia NH 4 (OH) and 37.2% by weight solids at 160°C for 30 minutes;
[0306] - aluminum doped silica having a doping level of 1% by weight; and
[0307] - an amount of silica of 50 phr when bringing the two dispersions into contact with each other.
[0308] In the procedure detailed above, the only difference between these four experiments was to vary the pH of the aqueous dispersion of doped silica to vary the pH of the formulation.
[0309] Thus, tests E1, E2, E3 and E4 differ from each other in the pH of t...
Embodiment 2
[0319] The purpose of this example is to demonstrate the suitable operation of the method according to the invention, in particular for the pH of the formulation measured at a silica doping level different from Example 1 .
[0320] According to the method described in detail in the previous section, experiments E'1, E'2, E'3 and E'4 were prepared with the same field latex as mentioned in Example 1 and with 2.5% by weight of Al-doped silica at a dopant level such that the amount of silica at which the two dispersions were in contact with each other was 50 phr.
[0321] As in Example 1, the only difference between these four experiments was the change of the pH of the aqueous dispersion of doped silica and thus the formulation pH while following the procedure detailed above, thus:
[0322] - in the case of E'1, the pH of the preparation is 3;
[0323] - in the case of E'2, the pH of the formulation is 5;
[0324] - in the case of E'3, the pH of the formulation is 7;
[0325] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com