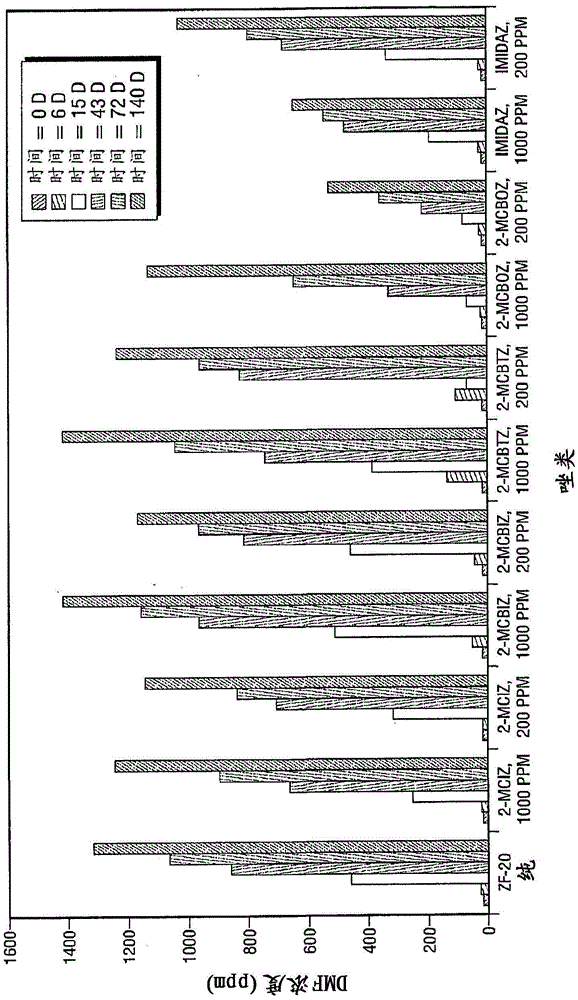
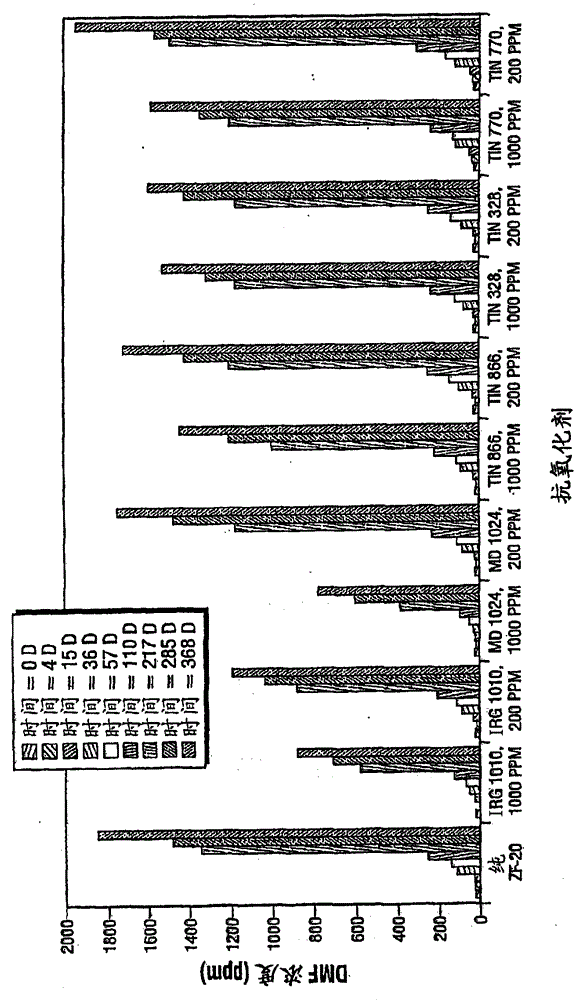
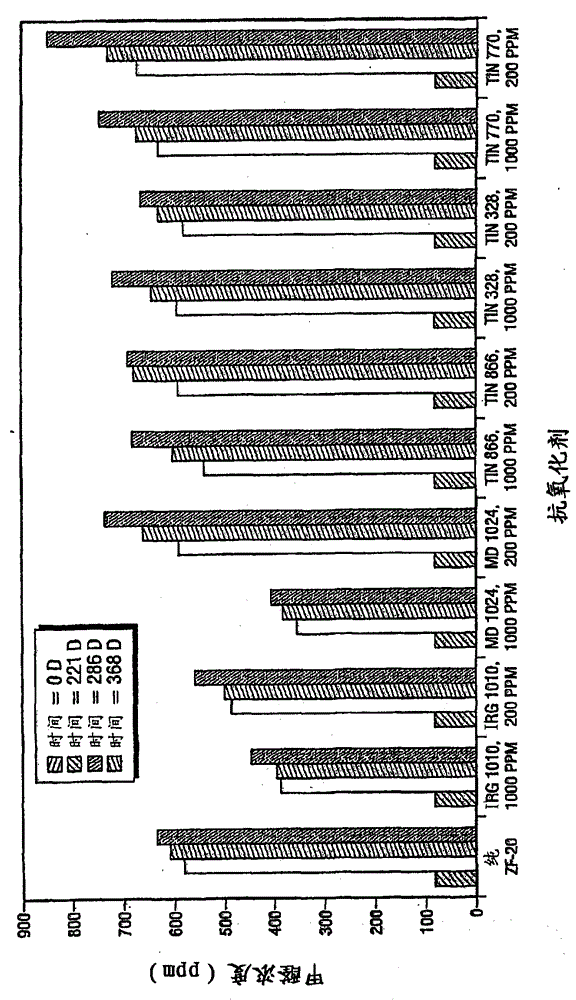
Inhibition of amine oxidation
A technology of amine oxidation and inhibitors, applied in the preparation of organic compounds, preparation of amino hydroxyl compounds, organic chemistry, etc., can solve problems such as allergic reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] In Examples 1a-1c, the concentrations of DMF and formaldehyde in samples mixed with tertiary amines and methimazole (MM) were recorded. Generally, bis(2-dimethylaminoethyl) ether (JEFFCAT ZF-20 amine catalyst, available from Huntsman Petrochemical LLC, The Woodlands, Texas) Prepare 50 ml of methimazole (amine oxidation inhibitor) solutions at 10, 100, 250 and 1000 ppm, respectively. Methimazole is commercially available from Sigma-Aldrich Corp., St. Louis, MO.
[0042] 8ml aliquots of each of the above formulations were poured into corresponding 20ml vials, and untreated JEFFCAT 8ml aliquots of ZF-20 amine catalyst were poured into separate 20ml vials. Thus, there were five 20ml vials in one set; at least one sample in each set contained 0, 10, 100, 250 or 1000 ppm methimazole. Sample sets were incubated at 25°C, 40°C, or 70°C. An aliquot (approximately 0.4 ml) of each sample was withdrawn periodically to test the concentration of DMF and / or formaldehyde formed in...
Embodiment 1a
[0044] In this example, the DMF and formaldehyde concentrations were recorded in a set of samples incubated at room temperature (approximately 25° C.) for up to 680 days. Sample 1a (pure ZF-20) is a comparative sample without methimazole, while Examples 2a, 3a, 4a and 5a are test samples that include 10, 100, 250 and 1000 ppm of methimazole, respectively. Before incubation, a baseline of DMF and formaldehyde concentrations was determined. Thereafter, the DMF and formaldehyde concentrations of each sample in the group were regularly determined.
[0045] refer to image 3 , shows the DMF concentration (ppm) of samples 1a-5a at different time points. The samples did not show appreciable differences in DMF accumulation after approximately 1 day (18 hours) of incubation. However, thereafter, the DMF concentration in sample 1a started to rise compared to the test sample, and at 12 days and beyond, the difference in DMF concentration between the comparative sample 1a and the test ...
Embodiment 1b
[0056] In this example, a set of samples was measured for the presence of DMF after a total of 39 days in an oven at 40°C. Sample 1b is a comparative sample without methimazole, while Examples 2b, 3b, 4b and 5b are test samples which include 10, 100, 250 and 1000 ppm of methimazole, respectively.
[0057] refer to Figure 4 , shows the DMF concentrations for samples 1a to 5b. Like Comparative Sample 1a, Comparative Sample 1b showed a steady increase in DMF concentration over 39 days. In contrast, samples 2b-5b had reduced DMF formation over time compared to comparative sample 1b. The most effective concentration of methimazole at 40°C is 1000ppm. Interestingly, after 18 days incubation at 40°C, sample 4b containing 250 ppm methimazole was not as effective as sample 3b containing 100 ppm methimazole.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com