Tartaric acid vinorelbine powder injection for injection and preparation method thereof
A technology for vinorelbine tartrate and tartaric acid, which is applied to the field of vinorelbine tartrate powder injection for injection and its preparation, can solve the problems of not examining the stability of the preparation, and achieve the effects of excellent appearance and good redissolving behavior
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
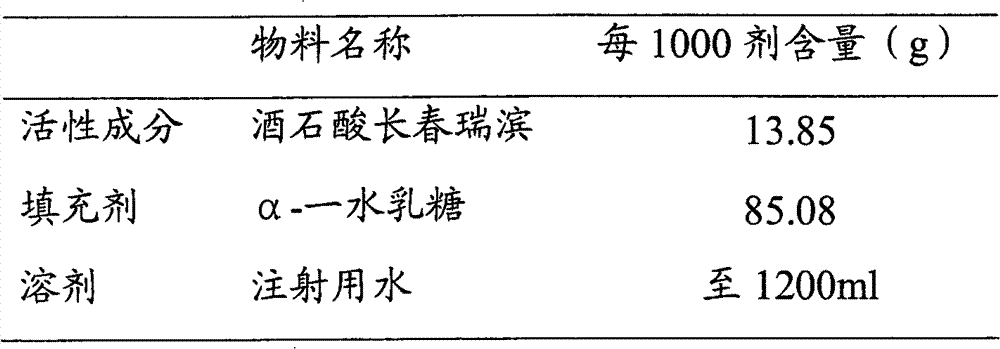
[0029] Embodiment 1 (vinorelbine tartrate 14%, α-lactose monohydrate 86%)
[0030]
[0031] ①Weigh α-lactose monohydrate, add appropriate amount of water for injection and stir until dissolved, then add water for injection to a sufficient amount; heat the lactose solution to 60±5°C.
[0032] Weigh the activated carbon with 0.25% (w / w) lactose solution, take an appropriate amount of lactose solution to moisten the activated carbon, add it, stir and keep it warm for 20 minutes, then add the second equal amount of activated carbon, stir and keep it warm for 20 minutes, and the adsorption is over.
[0033] The lactose solution is decarbonized and filtered through a filter element, and cooled to room temperature for later use.
[0034] ② Weigh the prescribed amount of vinorelbine tartrate, add appropriate amount of cooled lactose solution and stir until dissolved, make up to the full amount, after stirring evenly, take samples for content, color and other items testing; steriliz...
Embodiment 2
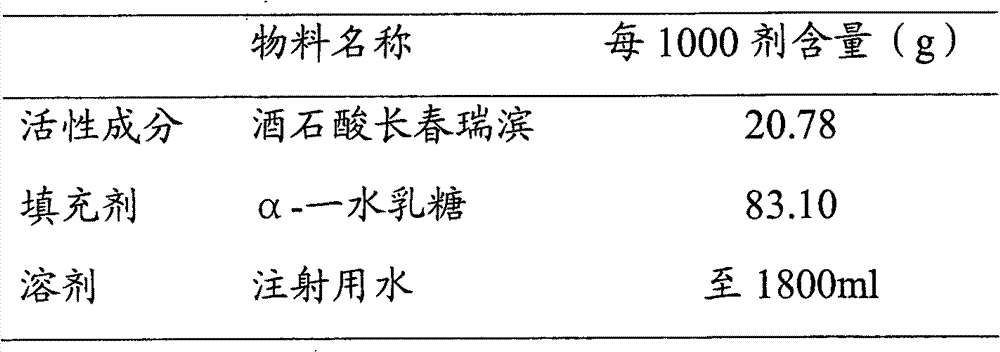
[0038] Embodiment 2 (vinorelbine tartrate 20%, α-lactose monohydrate 80%)
[0039]
[0040] ①Weigh α-lactose monohydrate, add appropriate amount of water for injection and stir until dissolved, then add water for injection to a sufficient amount; heat the lactose solution to 60±5°C.
[0041] Weigh the activated carbon with 0.25% (w / w) lactose solution, take an appropriate amount of lactose solution to moisten the activated carbon, add it, stir and keep it warm for 20 minutes, then add the second equal amount of activated carbon, stir and keep it warm for 20 minutes, and the adsorption is over.
[0042] The lactose solution is decarbonized and filtered through a filter element, and cooled to room temperature for later use.
[0043] ② Weigh the prescribed amount of vinorelbine tartrate, add appropriate amount of cooled lactose solution and stir until dissolved, make up to the full amount, after stirring evenly, take samples for content, color and other items testing; steriliz...
Embodiment 3
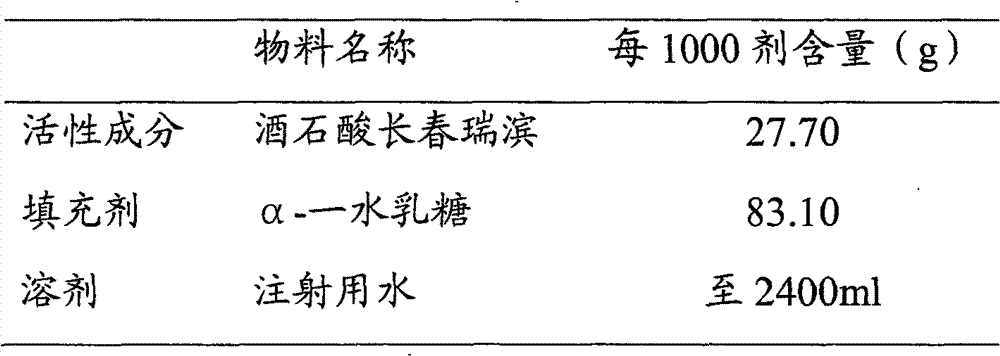
[0047] Embodiment 3 (vinorelbine tartrate 25%, α-lactose monohydrate 75%)
[0048]
[0049] ①Weigh α-lactose monohydrate, add appropriate amount of water for injection and stir until dissolved, then add water for injection to a sufficient amount; heat the lactose solution to 60±5°C.
[0050] Weigh the activated carbon with 0.25% (w / w) lactose solution, take an appropriate amount of lactose solution to moisten the activated carbon, add it, stir and keep it warm for 20 minutes, then add the second equal amount of activated carbon, stir and keep it warm for 20 minutes, and the adsorption is over.
[0051] The lactose solution is decarbonized and filtered through a filter element, and cooled to room temperature for later use.
[0052] ② Weigh the prescribed amount of vinorelbine tartrate, add appropriate amount of cooled lactose solution and stir until dissolved, make up to the full amount, after stirring evenly, take samples for content, color and other items testing; steriliz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com