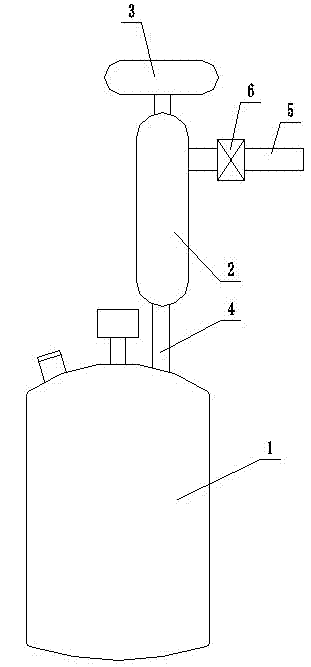
Preparation method for bromoalkane
A bromoalkane and crude product technology, which is applied in the field of bromoalkane preparation, can solve problems such as troublesome post-processing, complex catalyst synthesis and regeneration process affecting popularization, unstable yield of bromoalkane, etc., to achieve good product quality and production The effect of low cost and stable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 向450Kg 浓度为48%的氢溴酸中加入92 Kg无水乙醇,搅拌25分钟,生成混合料液,搅拌下加热混合料液达到回流状态,保持搅拌和回流状态使物料反应10小时,制得产物混合液A;在37-42℃温度条件下蒸馏产物混合液A,蒸出的气体经冷凝后制得产物混合液B;将产物混合液B静置35分钟使之分层,取下层液体制得溴乙烷粗品C 198.4公斤;向上述溴乙烷粗品C中加入200升水与50升4%的碳酸钠溶液,然后搅拌洗涤30分钟,得pH值为7.5的洗涤混合液,静置洗涤混合液使之分层,取出下层的液体得溴乙烷粗品D 188.3公斤,将溴乙烷粗品D加入到精馏塔中在温度38-41℃条件下进行精馏,蒸出的气体经冷凝后,便得到液体状的溴乙烷182.03公斤,按乙醇计收率为83.5%,溴乙烷的主含99.93%。
Embodiment 2
[0024] 向422Kg 浓度为48%的氢溴酸中加入60Kg无水正丙醇,搅拌35分钟,生成混合料液,搅拌下加热混合料液达到回流状态,保持搅拌和回流状态使物料反应12小时,制得产物混合液A;在71-76℃温度条件下蒸馏产物混合液A,蒸出的气体经冷凝后制得产物混合液B;将产物混合液B静置30分钟使之分层,取下层液体制得溴代正丙烷粗品C 114.4公斤;向上述溴代正丙烷粗品C中加入200升水与20升5%的碳酸钠溶液,然后搅拌洗涤30分钟,得pH值为8.1的洗涤混合液,静置洗涤混合液使之分层,取出下层的液体得溴代正丙烷粗品D 109公斤,将溴代正丙烷粗品D加入到精馏塔中在温度72-75℃条件下进行精馏,蒸出的气体经冷凝后,便得到液体状的溴代正丙烷104.66公斤,按正丙醇计收率为85.1%,溴代正丙烷的主含99.92%。
Embodiment 3
[0026] 向608Kg 浓度为48%的氢溴酸中加入60Kg无水异丙醇,搅拌30分钟,生成混合料液,搅拌下加热混合料液达到回流状态,保持搅拌和回流状态使物料反应12小时,制得产物混合液A;在58-63℃温度条件下蒸馏产物混合液A,蒸出的气体经冷凝后制得产物混合液B;将产物混合液B静置30分钟使之分层,取下层液体制得溴代异丙烷粗品C113.2公斤;向上述溴代异丙烷粗品C中加入200升水与30升5%的碳酸钠溶液,然后搅拌洗涤30分钟,得pH值为8.2的洗涤混合液,静置洗涤混合液使之分层,取出下层的液体得溴代异丙烷粗品D109.3公斤,将溴代异丙烷粗品D加入到精馏塔中在温度59-62℃条件下进行精馏,蒸出的气体经冷凝后,便得到液体状的溴代异丙烷106.08公斤,按异丙醇计收率为86.2%,溴代异丙烷的主含99.93%。
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com