Process for the reduction of cinnamaldehyde derivative employing enoate reductases
一种醛化合物、醚化合物的技术,应用在Z.Hamersak,,,K.Faber,Angew.Chem.Int.E领域
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
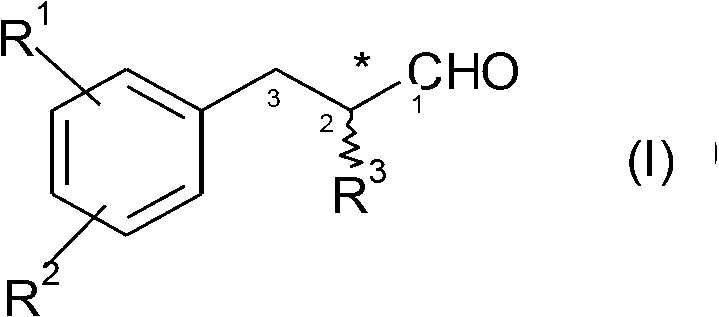
[0011] 1. In a first embodiment, the present invention provides a biocatalytic process, in particular an enzymatic catalytic process, for the production, in particular the asymmetric synthesis, of aldehyde compounds of general formula 1
[0012]
[0013] in
[0014] R 1 and R 2 represent independently of each other a linear or branched, optionally substituted alkyl group, such as C 1 -C 8 or C 1 -C 6 Alkyl; alkenyl, such as C 2 -C 8 or C 2 -C 6 Alkenyl; alkynyl, such as C 2 -C 8 or C 2 -C 6 Alkynyl; alkoxy, such as C 1 -C 8 or C 1 -C 6 Alkoxy; alkenyloxy, such as C 2 -C 8 or C 2 -C 6Alkenyloxy; -H, -OH, -SH, -halogen, such as F, Cl or Br; -NH 2 , or -NO 2 ;
[0015] In particular, H or linear or branched alkyl, such as C 1 -C 6 or C 1 -C 4 Alkyl; alkenyl, such as C 2 -C 6 Alkenyl; alkynyl, such as C 2 -C 6 Alkynyl; alkoxy, such as C 1 -C 6 or C 1 -C 4 Alkoxy; alkenyloxy, such as C 2 -C 6 Alkenyloxy; or, more specifically, H or branched C...
Embodiment 2
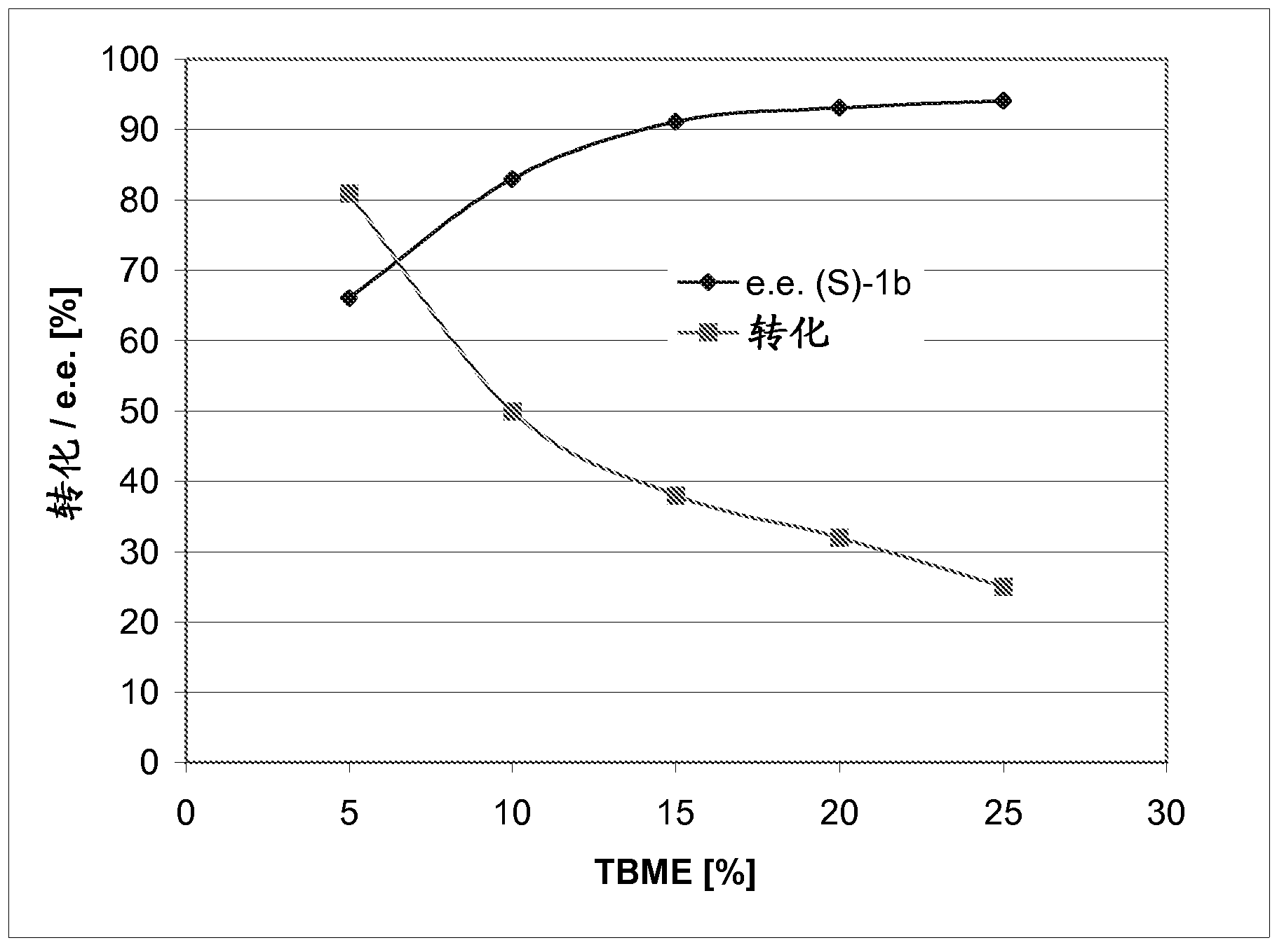
[0219] Embodiment 2: the bioreduction experiment that carries out with different cinnamaldehyde
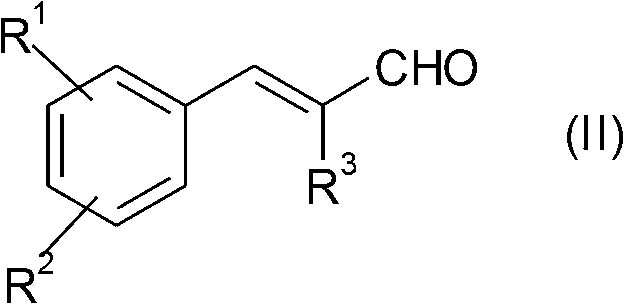
[0220] route figure 1 .Asymmetric bioreduction of α-methylcinnamaldehyde derivatives 1a-3a
[0221]
[0222] 2.1 Preparation experiment
[0223] (1) General method for bioreduction of enzymes under standard conditions
[0224] Add aliquots of the enzymes (OPR1, OPR3, YqjM, OYE1-3, NCR, NEM-reductase, protein concentration in biotransformation: 75-125 µg / mL) to the cells containing the substrate (10 mM) and the cofactor NADH. (10mM) Tris-HCl buffer (0.8mL, 50mM, pH 7.5). The mixture was shaken at 30°C and 120 rpm. After 24 h, the product was extracted with EtOAc (2 x 0.5 mL). in Na 2 SO 4 The combined organic phases were dried above and analyzed respectively on achiral GC to determine conversion and on chiral GC or on HPLC to determine enantiomeric excess.
[0225] (2) General method of cofactor recycling
[0226] Aliquots of the enzyme (see above) were added to the sub...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com