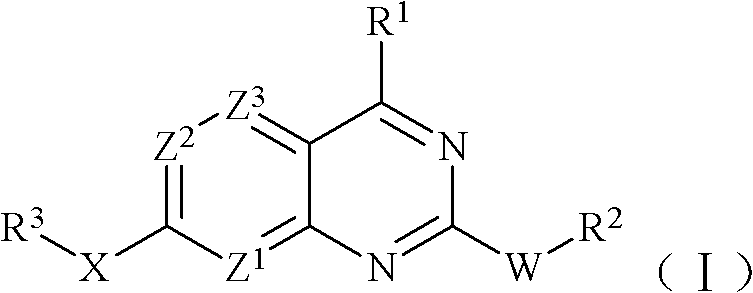
Pyridopyrimidine mammalian target of rapamycin (mTOR) inhibitor
A technology of alkyl and compound, applied in the field of pyridopyrimidine mTOR inhibitors, which can solve the problems of poor solubility and stability, large molecular weight, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
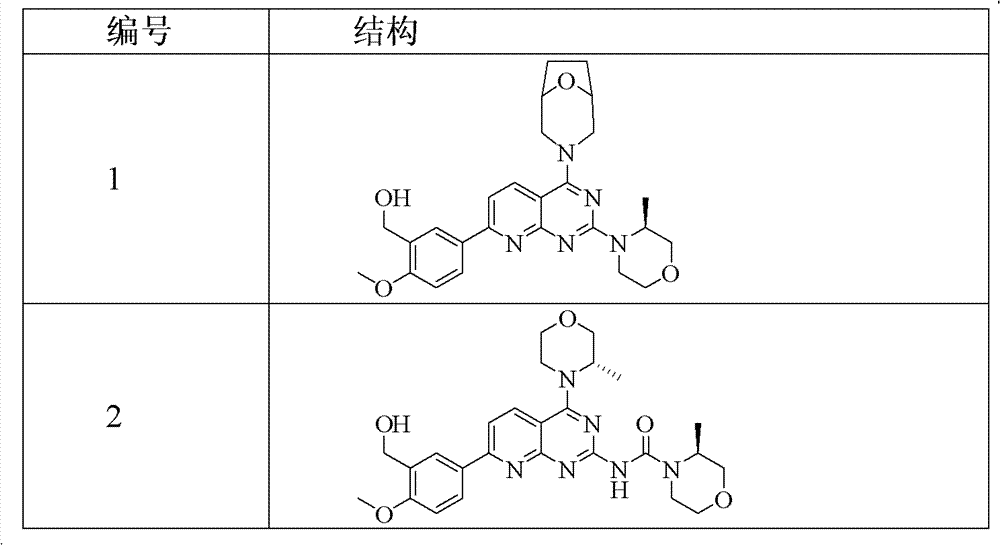
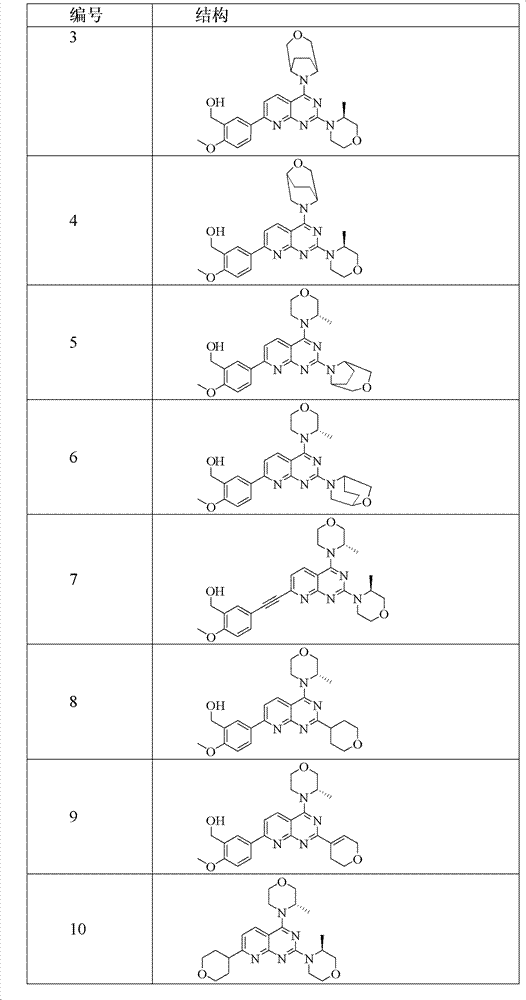
[0214] Example 1 (5-(4-(8-oxa-3-azabicyclo[3.2.1]nonan-3-yl)-2-((S)-3-methylmorpholine)pyrido[ 2,3-d] pyrimidine Preparation of pyridin-7-yl)-2-methoxyphenyl)methanol (compound 1)
[0215]
[0216] (1) 3-(2,7-dichloropyrido[2,3-d]pyrimidin-4-yl)-8-oxa-3-azabicyclo[3.2.1]nonane
[0217]
[0218] 2,4,7-trichloropyrido[2,3-d]pyrimidine (1g, 4.29mmol) and 8-oxa-3-azabicyclo[3.2.1]nonane hydrochloride (640mg, 4.29 mmol) was dissolved in 20 mL of DCM, and DIEA (0.9 mL, 5.15 mmol) was added dropwise in an ice-water bath. After the reaction was stirred at room temperature for 18 h, the reaction solution was washed with water, the organic layer was dried and concentrated to obtain a solid, which was directly used in the next reaction without purification.
[0219] (2)(5-(4-(8-oxa-3-azabicyclo[3.2.1]nonan-3-yl)-2-chloropyrido[2,3-d]pyrimidin-7-yl )-2-methoxyphenyl)methanol
[0220]
[0221] 3-(2,7-dichloropyrido[2,3-d]pyrimidin-4-yl)-8-oxa-3-azabicyclo[3.2.1]nonane (310...
Embodiment 2
[0227] Example 2 (5-(4-(3-oxa-8-azabicyclo[3.2.1]nonan-8-yl)-2-((S)-3-methylmorpholine)pyrido[ 2,3-d]pyrimidine-7- base)-2-methoxyphenyl)methanol preparation (compound 3)
[0228]
[0229] (1) 8-(2,7-dichloropyrido[2,3-d]pyrimidin-4-yl)-3-oxa-8-azabicyclo[3.2.1]nonane
[0230]
[0231] Operation is the same as in Example 1(1), and the product is directly used in the next step without purification.
[0232] (2)(5-(4-(3-oxa-8-azabicyclo[3.2.1]nonan-8-yl)-2-chloropyrido[2,3-d]pyrimidin-7-yl )-2-methoxyphenyl)methanol
[0233]
[0234] Operation is the same as in Example 1(2), and the product is directly used in the next step without purification.
[0235] (3) (5-(4-(3-oxa-8-azabicyclo[3.2.1]nonan-8-yl)-2-((S)-3-methylmorpholine)pyridine[2 ,3-d]pyrimidin-7-yl)-2-methoxyphenyl)methanol
[0236]
[0237] Operation is the same as Example 1(3), and the productive rate is 7.5%.
[0238] Molecular formula: C 26 h 31 N 5 o 4 Molecular weight: 477.5 Mass spectr...
Embodiment 3
[0240] Example 3 (5-(4-(2-oxa-5-azabicyclo[2.2.2]nonan-5-yl)-2-((S)-3-methylmorpholine)pyrido[ 2,3-d]pyrimidine-7- base)-2-methoxyphenyl)methanol preparation (compound 4)
[0241]
[0242] (1) 5-(2,7-dichloropyrido[2,3-d]pyrimidin-4-yl)-2-oxa-5-azabicyclo[2.2.2]nonane
[0243]
[0244]Operation is the same as in Example 1(1), and the product is directly used in the next step without purification.
[0245] (2)(5-(4-(2-oxa-5-azabicyclo[2.2.2]nonan-5-yl)-2-chloropyrido[2,3-d]pyrimidin-7-yl )-2-methoxyphenyl)methanol
[0246]
[0247] Operation is the same as in Example 1(2), and the product is directly used in the next step without purification.
[0248] (3) (5-(4-(2-oxa-5-azabicyclo[2.2.2]nonan-5-yl)-2-((S)-3-methylmorpholine)pyrido[ Preparation of 2,3-d]pyrimidin-7-yl)-2-methoxyphenyl)methanol
[0249]
[0250] Operation is the same as Example 1(3), and the productive rate is 29%.
[0251] Molecular formula: C 26 h 31 N 5 o 4 Molecular weight: 477.5 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com