Neutralizing antibody of EB (Epstein-Barr) virus and application thereof
A technology of Epstein-Barr virus and bispecific antibody, which is applied in the fields of application, antibody, antiviral agent, etc., can solve the problems of late start of bispecific antibody, and achieve the effects of inhibiting growth and metastasis, reducing titer, and high affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1: Preparation of EBVgp350 monoclonal antibody of the present invention
[0064] This example describes the generation of high affinity neutralizing monoclonal antibodies against the glycoprotein gp350 of Epstein-Barr virus.
[0065] Two New Zealand white rabbits about 6 weeks old (about 2kg in weight) were immunized with recombinant gp350 protein every 14 days. After 3 times of immunization, the spleens of the rabbits were collected, the total RNA in the spleen was extracted, and reverse-transcribed into cDNA. A phage-displayed antibody library was constructed, and monoclonal R1 and R9 that specifically bind gp350 were obtained after four rounds of affinity screening.
[0066] The heavy chain variable region amino acid sequence of the R1 antibody is shown in SEQ ID NO: 1, and the light chain variable amino acid sequence is shown in
[0067] Shown in SEQ ID NO:2.
[0068] The amino acid sequence of the heavy chain variable region of the R9 antibody is shown i...
Embodiment 2
[0072] Example 2: Determination of the binding activity of the monoclonal antibody R1 of the present invention
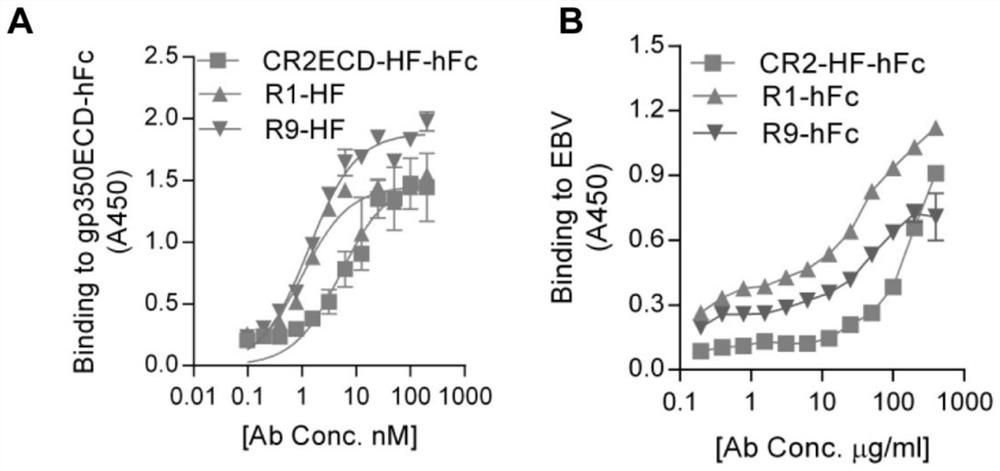
[0073] The scFv of R1 / R9 monoclonal antibody was fused to FLAG tag sequence or hFc and expressed in mammalian cell 293F. After the expression product was purified, the binding of the purified antibody to gp350 protein and EBV virus particles was tested by sandwich ELISA method. Coat gp350-hFc or EBV granule protein on the ELISA plate, add monoclonal antibody R1 / R9 at serial dilution for incubation, and use HRP-mouse anti-FLAG or HRP-goat anti-human antibody to detect R1 / R9, gp350 and EBV binding capacity ( figure 1 ). The results show that R1, R9 and CR2 all have high affinity with recombinant protein gp350 and EBV particles, and the binding activity of R1 is better than that of R9 and CR2
Embodiment 3
[0074] Example 3: Virus neutralizing activity test of the monoclonal antibody R1 of the present invention
[0075] 1. Monoclonal antibody R1 / R9 of the present invention blocks the binding activity of gp350 and CR2
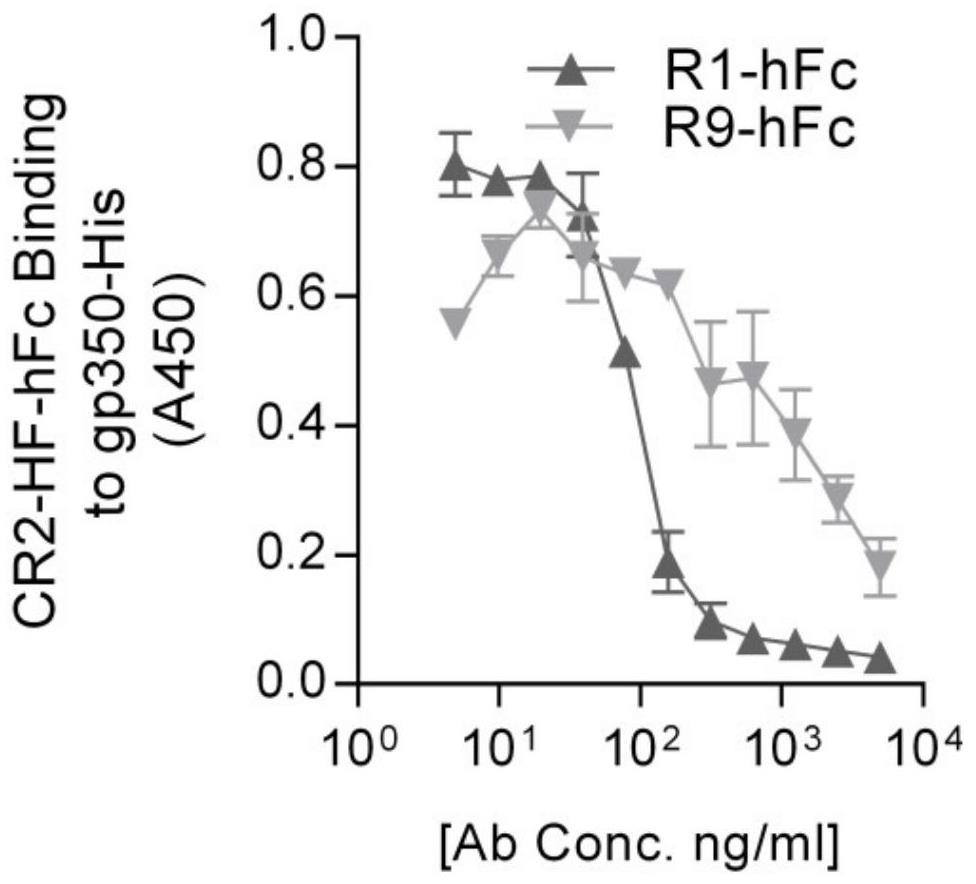
[0076] ELISA verified the neutralizing activity of the monoclonal antibody in vitro. Coat gp350-hFc on the bottom of the plate, add serially diluted R1-hFc for binding, add 2 μg / ml recombinant CR2 protein, and determine whether the antibody can effectively block the combination of the two by detecting the binding of CR2. The results show that R1 / R9 can effectively block the binding of CR2 and gp350, and the neutralizing activity of R1 is better than that of R9 ( figure 2 ).
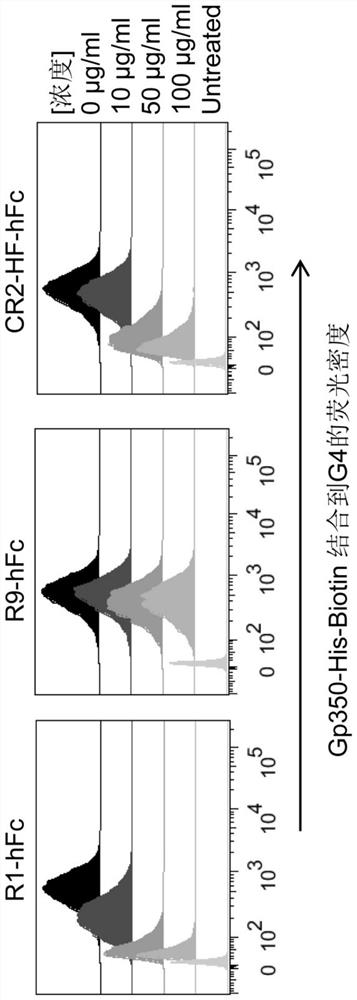
[0077] R1 / R9 blocks the binding of gp350 and CR2 by flow cytometry. The CR2-overexpressing cell line G4 was constructed on the basis of CR2-negative epithelial cells A431. Label gp350-His with biotin, then co-incubate with G4, add different concentrations of R1 antibody to block, and fin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com