Cationic electromigration imidazoline quaternary ammonium salt and application thereof
A kind of permanent imidazoline and cationic technology, which is applied in cationic electromobility imidazoline quaternary ammonium salt and in the field of concrete reinforcement protection, can solve the problems of slow migration speed, dangerous secondary corrosion, and high labor intensity. Achieve the effects of avoiding secondary corrosion, stable chemical properties, and low labor intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
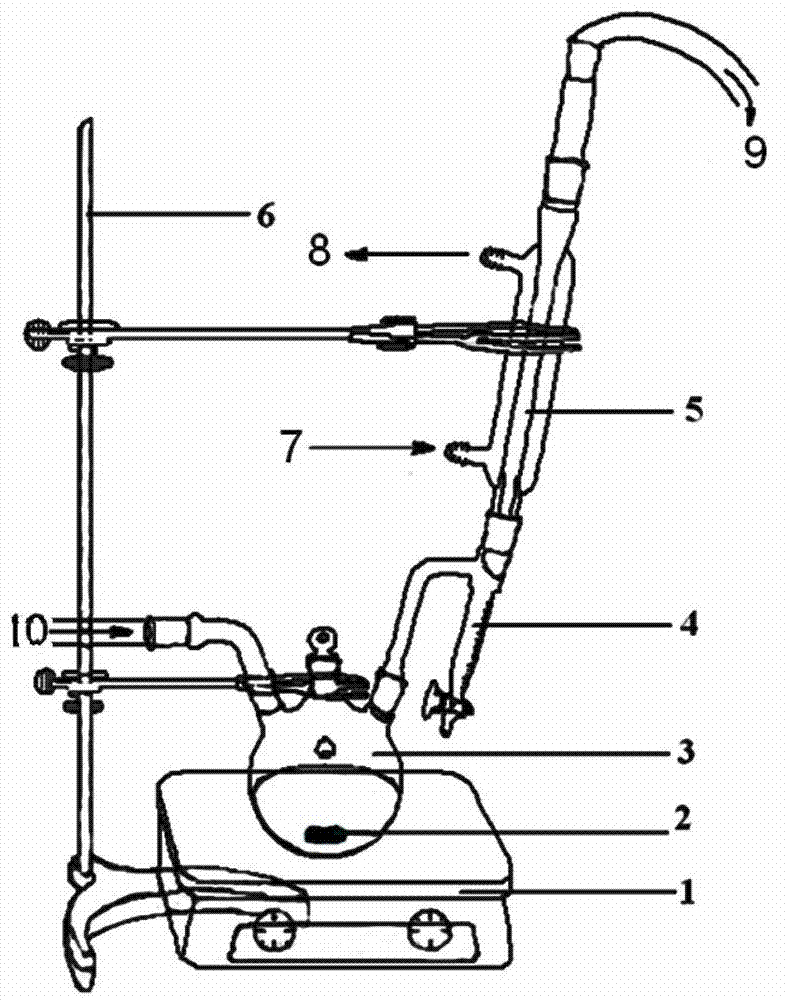
[0029] like figure 1 As shown, the device for preparing cationic electromobility imidazoline quaternary ammonium salt in this embodiment includes an iron stand 6, a three-necked flask 3, a collector type constant temperature heating magnetic stirrer 1, and also includes a water separator 4 and a condenser 5; The first flask mouth of the three-necked flask 3 is connected with one end of the water separator 4, and the condenser 5 is arranged on the water separator 4 and connected with the other end of the water separator 4; the second of the three-necked flask 3 The mouth of the flask is a nitrogen gas inlet 10, which is connected with the nitrogen supply pipe, and the mouth of the third flask of the three-necked flask 3 is sealed; xylene is housed in the water separator 4, and the liquid level of the xylene is lower than the extension of the water separator. Out of the lower end of the elbow 5mm.
[0030] The method for preparing cationic electromobility imidazoline quaternary...
Embodiment 2
[0052] This embodiment adopts the same device for preparing cationic electromobility imidazoline quaternary ammonium salt as in the embodiment, and the steps of the preparation method are as follows:
[0053] (1) Using lauric acid and diethylenetriamine at a molar ratio of 1.2:1, acylation reaction at 170°C for 3 hours, followed by cyclization reaction at 200°C for 15 hours, to synthesize imidazoline; into nitrogen.
[0054] (2) The molar ratio of imidazoline and epichlorohydrin is 1.0:1.0, and the cationic reaction is carried out at a temperature of 90 ° C and a pH value of 4. During the reaction, nitrogen gas is introduced, and the reaction time is 7 hours to obtain a cationic Electromobile imidazoline quaternary ammonium salt with the following molecular structure:
[0055]
[0056] Among them, R, R'represent CH 3 (CH 2 ) 10 -, -CH 2 CH 2 NHCO (CH 2 ) 10 CH 3 .
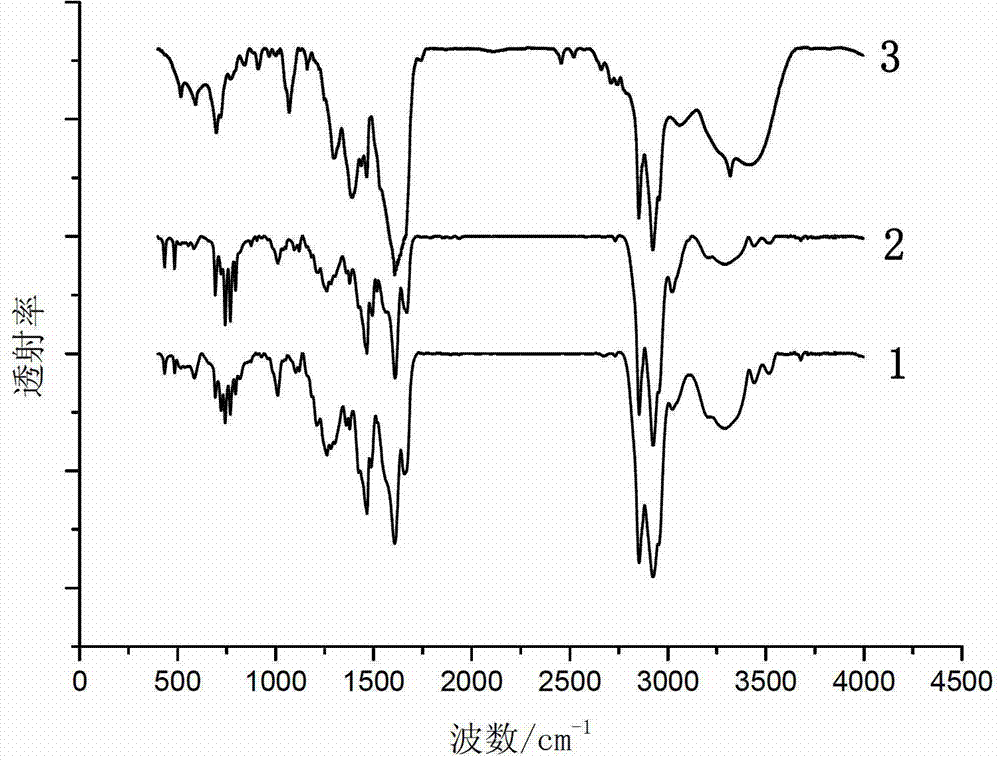
[0057] The infrared spectrogram of the imidazoline prepared by the step (1) of the present embodime...
Embodiment 3
[0060] This embodiment adopts the same device for preparing cationic electromobility imidazoline quaternary ammonium salt as in the embodiment, and the steps of the preparation method are as follows:
[0061] (1) Using lauric acid and diethylenetriamine at a molar ratio of 1:1.1, acylation reaction at 150°C for 4 hours and cyclization reaction at 210°C for 17 hours were used to synthesize imidazoline; into nitrogen.
[0062] (2) The molar ratio of imidazoline and ethylene oxide is 1:1.1, and the cationization reaction is carried out at a temperature of 70 ° C and a pH value of 3 to 4. During the reaction, nitrogen gas is introduced, and the reaction time is 8 hours. Cationic electromobile imidazoline quaternary ammonium salt with the following molecular structure:
[0063]
[0064] Among them, R, R'represent CH 3 (CH 2 ) 10 -, -CH 2 CH 2 NHCO (CH 2 ) 10 CH 3 .
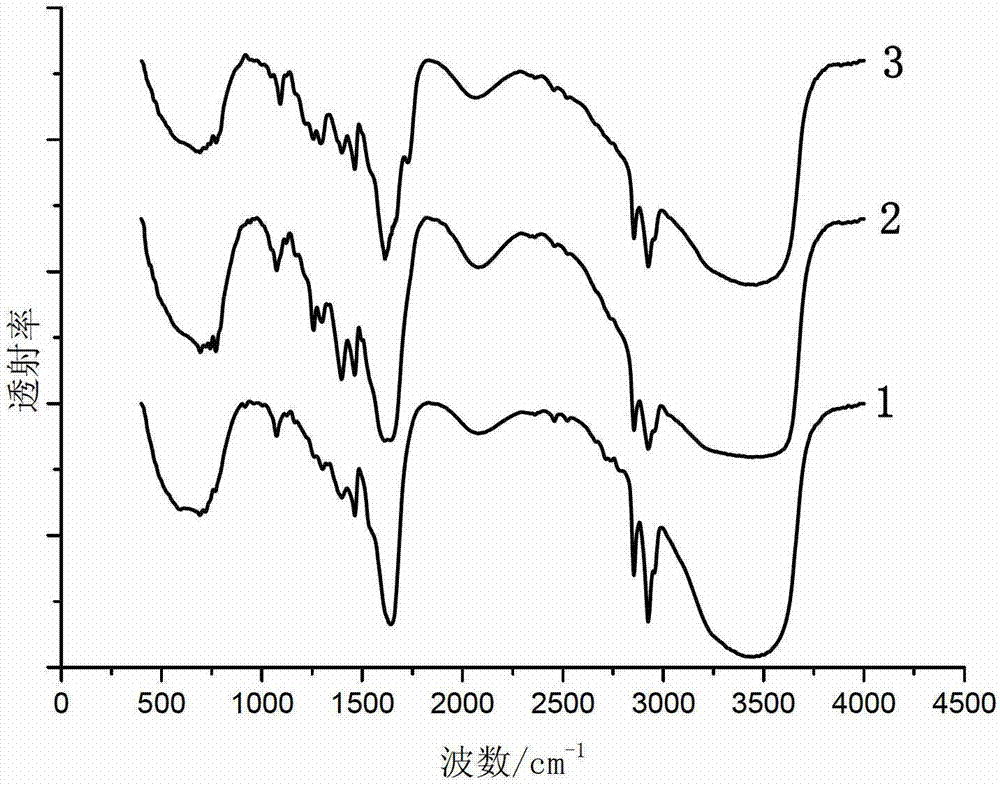
[0065] The infrared spectrogram of the imidazoline prepared by the step (1) of the present embodiment i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com