Oral film formulation
A technology of oral film and reducing agent, which is applied in drug delivery, sheet delivery, pharmaceutical formulation, etc., and can solve the problems of unsatisfactory chemical stability, ineffectiveness, and hindering the quality of triptan-containing pharmaceutical compositions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
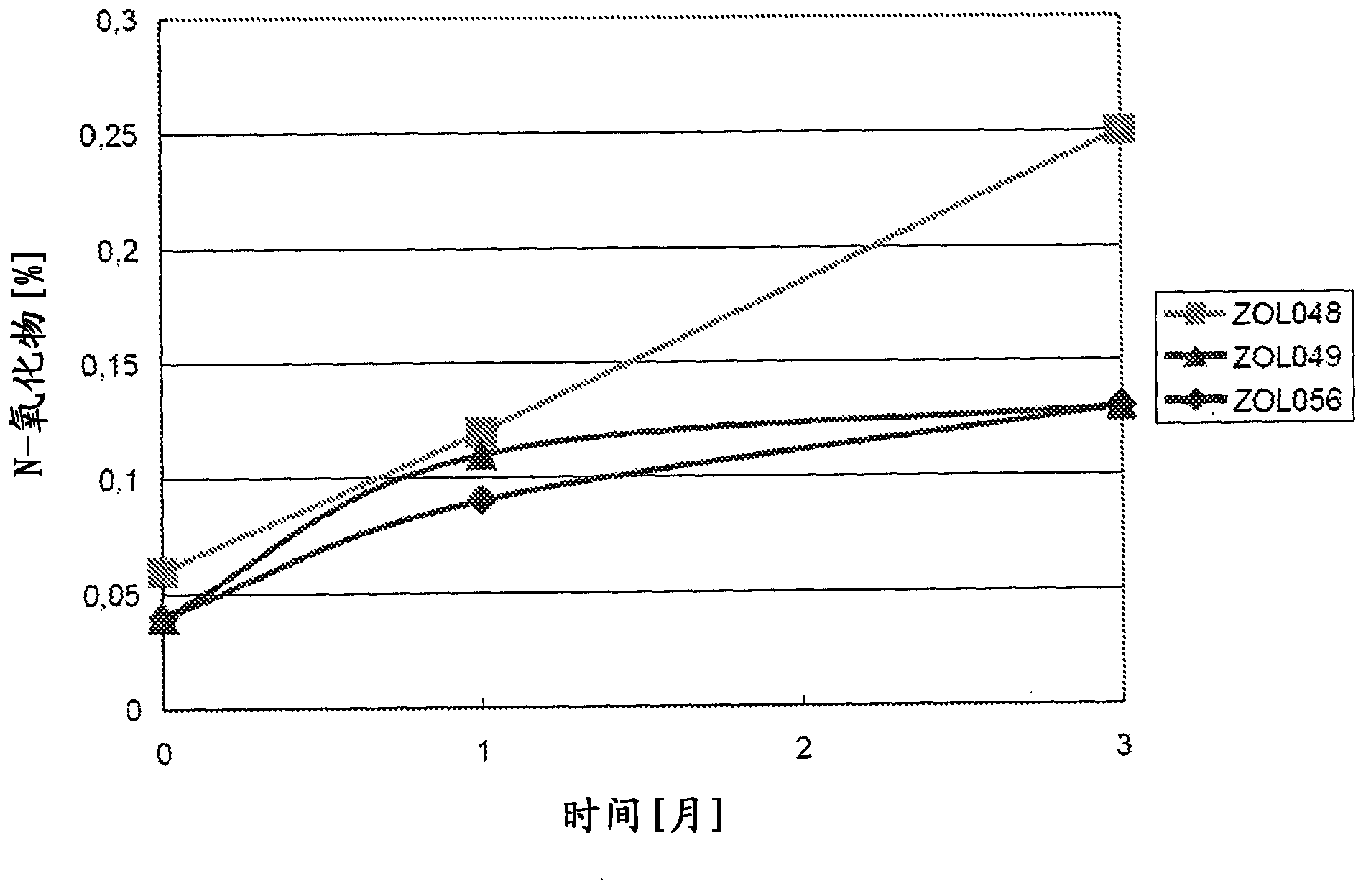
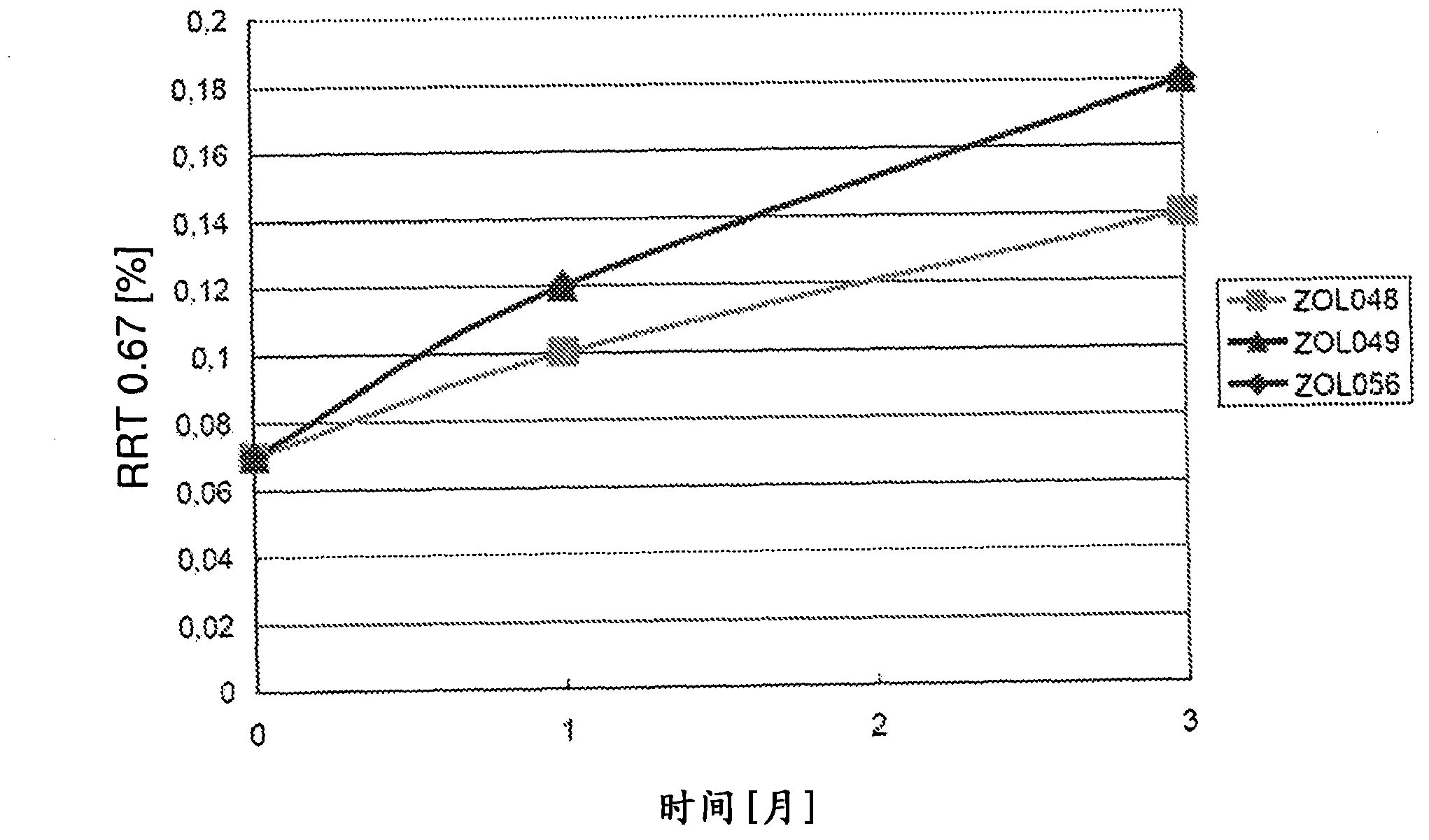
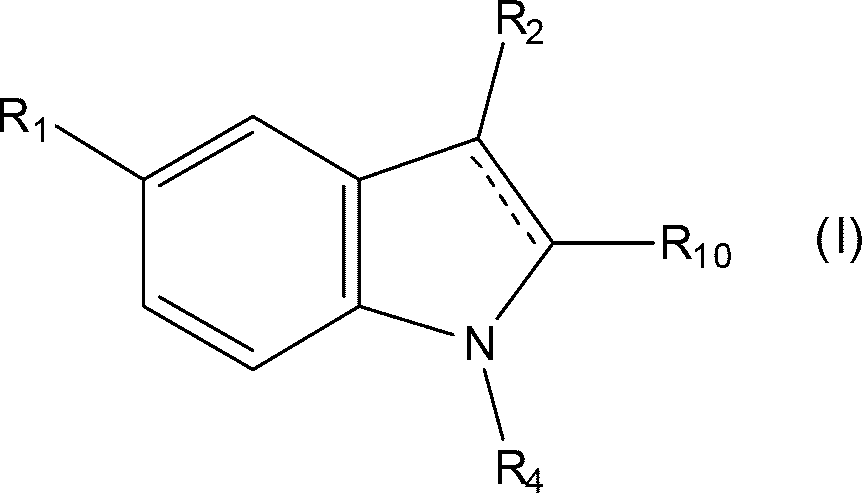
[0031] As stated above, the present invention relates to triptan-containing pharmaceutical compositions with increased triptan stability, ie reduced levels of triptan degradation products. The stability of triptans is increased due to the presence of thiol-containing substances. In one embodiment of the present invention, the concentration of the thiol-containing substance in the pharmaceutical composition is about 0.01 to about 7 wt%. Preferably, the mercapto-containing species is present at 0.07 to 6 wt%. In a more preferred embodiment, the concentration of the thiol-containing reducing agent is 0.2 to 1.0 wt%. In one embodiment, the concentration does not exceed 0.5 wt%.
[0032]According to the present invention, the relative amount of degradation products of triptans to the total amount of initial triptans can be reduced to a maximum of about 1%, that is, at least 99% of triptans can be recovered. This applies after the pharmaceutical composition has been "stress teste...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com