Method for enhancing reproductive capacity of CIK (Cytokine Induced Killer) cell and improving tumor cell killing capacity
A technology of cell proliferation and ability, applied in animal cells, vertebrate cells, blood/immune system cells, etc., can solve the problems of low number and activity of CIK cells, low tumor cell killing rate, poor therapeutic effect, etc., to improve cell Toxic effect, the effect of enhancing the growth ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Aseptically collect peripheral blood from healthy people and send it back to the cell preparation laboratory for experiments in a clean bench; separate mononuclear cells with lymphatic separation fluid, wash twice, resuspend cells with Q007 medium, and adjust the cell density at 3*10 6 / ml, and then follow the steps and methods below to culture CIK cells and control experiments.
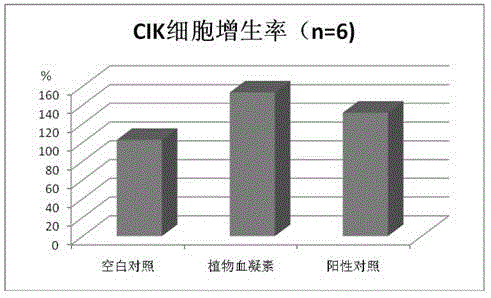
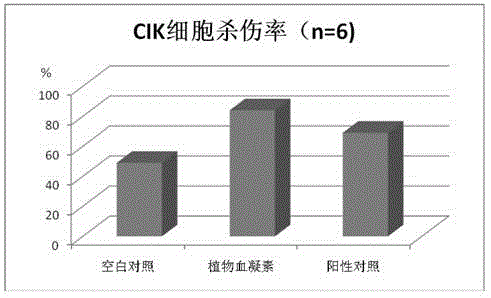
[0024] The experiment was divided into a blank group, a phytohemagglutinin group and a positive control CD3McAb group. The design scheme is as follows:
[0025] The experiment was carried out in a 12-well plate, and the inoculum volume in each well was 1ml
[0026] Blank control group:
[0027] Day 0: IFN-γ (250IU / ml)
[0028] Day 1: IL-1 (2.5ng / ml)
[0029] Day 2: IL-2 (1000IU / ml)
[0030] Remaining days: IL-2 (1000IU / ml)
[0031] Positive control group:
[0032] Day 0: IFN-γ (250IU / ml)
[0033] Day 1: CD3McAb (50ng / ml) 20ul, IL-1 (2.5ng / ml)
[0034] Day 2: IL-2 (1000IU / ml)
[0035] ...
Embodiment 2
[0053] Aseptically collect peripheral blood from healthy people and send it back to the cell preparation laboratory for experiments in a clean bench; use lymph separation fluid to separate mononuclear cells, resuspend the cells with Q007 medium, adjust the cell density to 2*106 / ml, and then The CIK cell culture and experimental control were carried out according to the following steps and methods.
[0054] The experiment was divided into a blank group, a phytohemagglutinin group and a positive control CD3McAb group. The design scheme is as follows:
[0055] The experiment was carried out in a 12-well plate, and the inoculum volume in each well was 1ml
[0056] Blank control group:
[0057] Day 0: IFN-γ (250IU / ml)
[0058] Day 1: IL-1 (2.5ng / ml)
[0059] Day 2: IL-2 (1000IU / ml)
[0060] Remaining days: IL-2 (1000IU / ml)
[0061] Positive control group:
[0062] Day 0: IFN-γ (250IU / ml)
[0063] Day 1: CD3McAb (50ng / ml) 20ul, IL-1 (2.5ng / ml)
[0064] Day 2: IL-2 (1000IU / ml...
Embodiment 3
[0074] Aseptically collect peripheral blood from healthy people and send it back to the cell preparation laboratory for experiments in a clean bench; separate mononuclear cells with lymphatic separation fluid, wash twice, resuspend cells with Q007 medium, and adjust the cell density at 3*10 6 / ml, and then follow the steps and methods below to culture CIK cells and control experiments.
[0075] The experiment was divided into a blank group, a phytohemagglutinin group and a positive control CD3McAb group. The design scheme is as follows:
[0076] The experiment was carried out in a 12-well plate, and the inoculum volume in each well was 1ml
[0077] Blank control group:
[0078] Day 0: IFN-γ (250IU / ml)
[0079] Day 1: IL-1 (2.5ng / ml)
[0080] Day 2: IL-2 (1000IU / ml)
[0081] Remaining days: IL-2 (1000IU / ml)
[0082] Positive control group:
[0083] Day 0: IFN-γ (250IU / ml)
[0084] Day 1: CD3McAb (50ng / ml) 20ul, IL-1 (2.5ng / ml)
[0085] Day 2: IL-2 (1000IU / ml)
[0086] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com