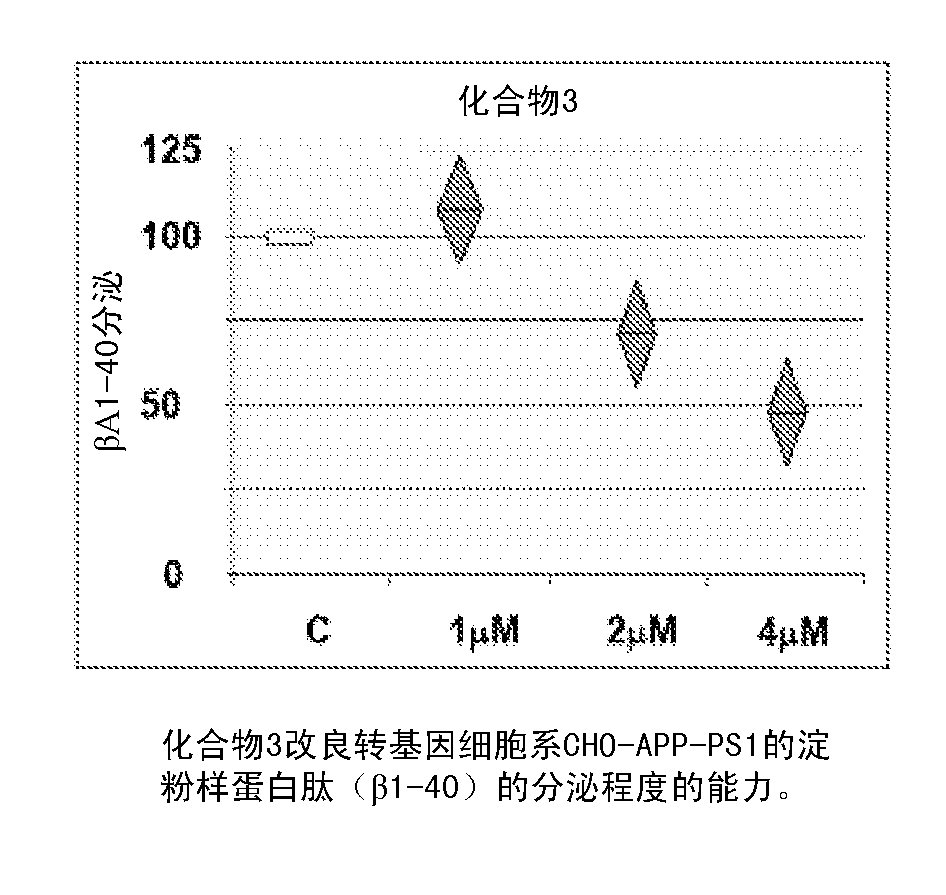
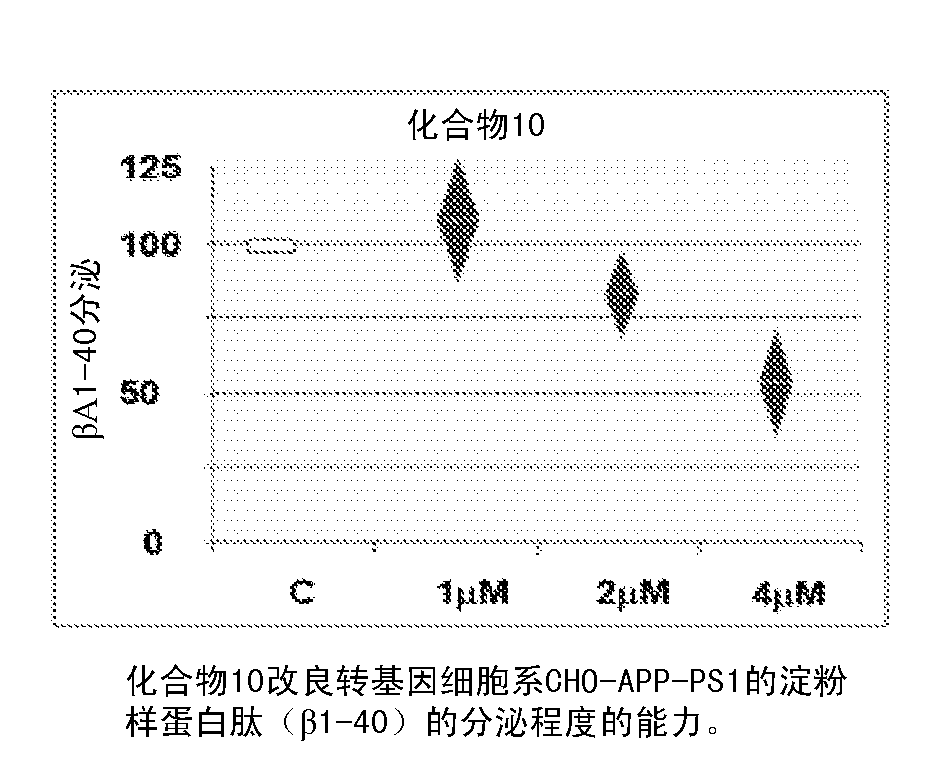
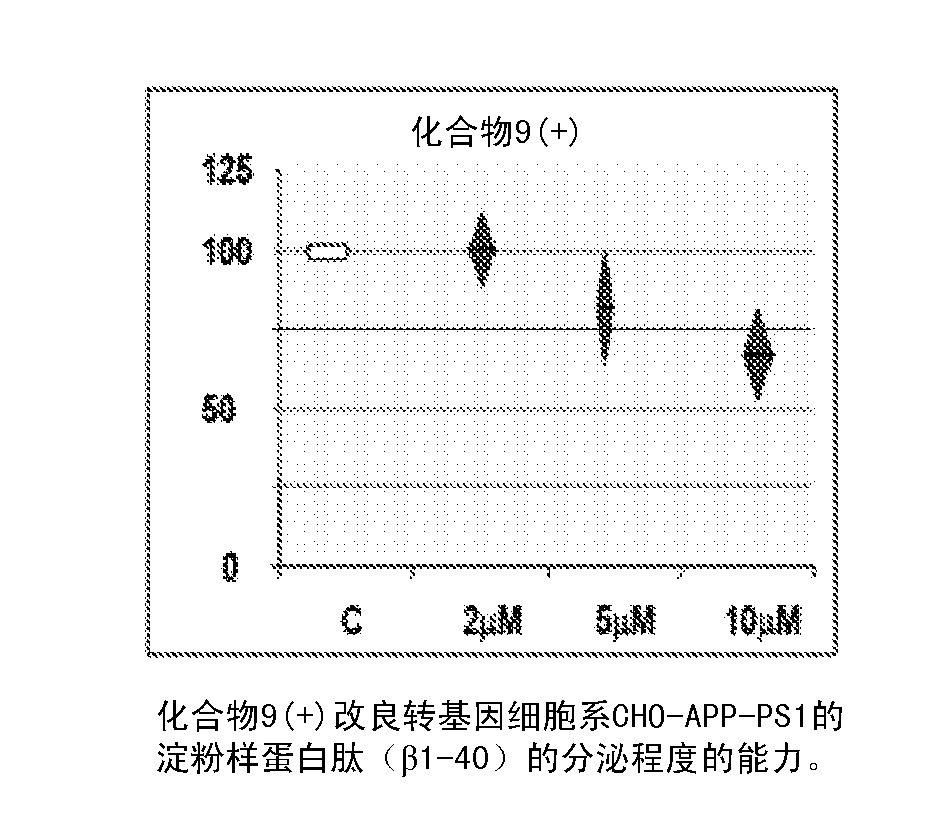
Flufenoxine derivatives for the treatment and prevention of amiloyd pathologies
A technology for pathological states and symptoms, applied in the field of flufenoxine derivatives, can solve problems such as no revelation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0065] According to one embodiment of the present invention, A is -C(H)- and B is -O-.
[0066] According to one embodiment of the present invention, C is a phenyl group optionally substituted with one or more groups independently selected from: -F, -Cl, -Br, -I, C 1 -C 3 Alkyl (such as methyl), C 1 -C 3 Alkenyl, C 1 -C 3 Perfluoroalkyl, -O-C 1 -C 3 -Alkyl (such as methoxy), -O-C 1 -C 3 -Alkenyl, -CN, C 6 -C 10 Aryl, C 7 -C 10 Aralkyl, wherein the C 1 -C 3 Alkyl and the O-C 1 -C 3 The alkyl group of the alkyl group can be -O-C 6 -C 10 Heteroaryl substitution. According to another embodiment, C is a phenyl substituted at least with -F, -Cl, -Br or -I, in particular a phenyl substituted with -F or -Cl.
[0067] According to another embodiment, C is a phenyl group of formula (VII)
[0068]
[0069] Of which halogen, R 3 And m are as defined above.
[0070] According to one embodiment of the invention, Ar is phenyl. According to an embodiment of the present invention, Ar is selected from...
specific Embodiment approach
[0135] Can follow in N 0 The general methods described in 9802420, EP1002794A1, US 6,518,284 or PCT / EP2007 / 053582 prepare the compounds of the present invention, including compounds of formula (II), (III), (IV) or (V).
[0136] And, according to the following plan 1, by using Me 3 S(O)I or MeOCH 2 PPh 3 (P.J.Gilliagan et al. J.Med.Chem.1992,35,23,4349) treat the corresponding ketone, and then reduce it to prepare the compound of formula (V) or related compounds. The resulting alcohol can be alkylated with the corresponding benzyl or phenyl derivatives.
[0137]
[0138] plan 1
[0139] Representative examples will be given below.
Embodiment 1
[0141] (+ / -)-4-[(naphthalene-1-oxy)(4-chlorophenyl)methyl]piperidine hemisulfate compound 31
[0142] The following procedure is similar to the preparation of compound 15au described in J. Med. Chem. 2003. Vol 46, 25, 5512-5532.
[0143] J Med 2003, vol 46, 25, 5512-5532 compound 14b (1.6g, 4.8mmol) was added in multiple portions to hexane washed NaH (0.2g 60% oil dispersion) / 10ml anhydrous DMSO in a stirred suspension. The reaction was stirred at room temperature for 30 minutes, potassium benzoate (0.7 g) was added, and stirring continued for 30 minutes, naphthalene (5.9 mmol) was added, and the reaction mixture was heated at 85°C for 15 hours, and then cooled to room temperature. A mixture of water and brine was added and the oil was extracted with ether. The organic layer was concentrated in vacuo, stirred with methanol (35 ml) and 10% aqueous HCl and refluxed for 1.5 hours. After removing the solvent, the residue is partitioned into 10% aqueous HCl and CHCl 3 between. Treat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com