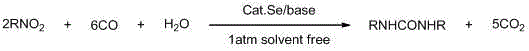Method for synthesizing symmetric urea compounds from nitrocompounds
A nitro compound and compound technology, applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve the problems of harsh reaction conditions, highly toxic phosgene, corrosive chlorides, etc., and reduce the reaction cost. , The effect of easy subsequent separation and less equipment investment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add nitrobenzene (2.46g), selenium powder (0.032g), H 2 O (0.18ml), 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) (0.76g), continue to feed carbon monoxide, then heat to 95°C, under normal pressure , stirred for 8 hours, switched carbon monoxide to air or oxygen for 0.5 to 1 hour to oxidize the selenium powder, cooled to room temperature, recrystallized the product with ethanol, and dried in vacuum to obtain diphenylurea. The purity of the product as measured by proton nuclear magnetic resonance is 100%, and the yield is 64%.
Embodiment 2
[0024] Experimental method and condition are the same as example 1, only change temperature, and the productive rate of diphenylurea is as follows:
[0025] temperature(℃) 95 85 75 65 55 45 35 Yield(%) 64 56 43 39 37 30 Trace
Embodiment 3
[0027] Experimental method and condition are the same as example 1, only change time, and the productive rate of diphenylurea is as follows:
[0028] time (h) 10 8 6 4 2 1 Yield(%) 64 64 58 44 40 34
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com