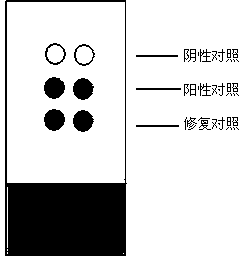
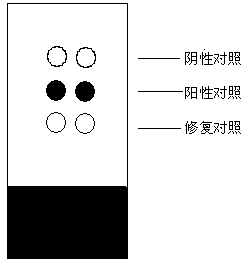
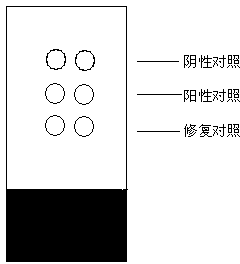
Immunohistochemical quality control reference object and quality control method
A technology of immunohistochemistry and reference materials, which is applied in the direction of biological testing, measuring devices, and preparation of test samples. It can solve the problems of providing and difficult to detect tissues, and achieve consistent quality control signals, long-term stability, and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The preparation steps of the negative control material in the present invention are as follows: Weigh 0.2g of skimmed milk powder, dissolve it in 10mL of double distilled water, and fully stir to dissolve. Weigh 0.2 g of dextran and add it to the above solution, after stirring, add 20 μL of glacial acetic acid, stir continuously to dissolve the dextran completely, and place it at 4°C to remove the air bubbles in the dextran solution. Beat the dextran solution into the sodium hydroxide solution with a disposable injection needle to solidify, forming white solid particles. The obtained solid particles were subjected to the same procedures as pathological tissues, dehydrated with gradient alcohol (dehydration steps: 80%, 90%, 95%, 100% ethanol with various concentrations were dehydrated for 2 hours), transparent with xylene, soaked in paraffin , and finally made into paraffin specimens by paraffin embedding. Slice and mount according to the same procedure as pathological ...
Embodiment 1
[0035] Embodiment 1, the preparation of negative control material
[0036] Weigh 0.2g of skimmed milk powder, dissolve in 10mL of double distilled water, stir well to dissolve. Weigh 0.2 g of dextran and add it to the above solution, after stirring, add 20 μL of glacial acetic acid, stir continuously to dissolve the dextran completely, and place it at 4°C to remove the air bubbles in the dextran solution. Beat the dextran solution into the sodium hydroxide solution with a disposable injection needle to solidify, forming white solid particles. The obtained solid particles were subjected to the same procedures as pathological tissues, dehydrated with gradient alcohol (dehydration steps: 80%, 90%, 95%, 100% ethanol with various concentrations were dehydrated for 2 hours), transparent with xylene, soaked in paraffin , and finally made into paraffin specimens by paraffin embedding. Slice and mount according to the same procedure as pathological tissue.
Embodiment 2
[0037] Embodiment 2, the preparation of positive control material
[0038] Measure 10 mL of double distilled water, add antigen protein with a final concentration of 0.1 mg / L and fully dissolve it. Weigh 0.2g of skimmed milk powder, dissolve it in the above antigen solution, stir well to dissolve. Weigh 0.2 g of dextran and add it to the above solution, after stirring, add 20 μL of glacial acetic acid, stir continuously to dissolve the dextran completely, and place it at 4°C to remove the air bubbles in the dextran solution. Beat the dextran solution into the sodium hydroxide solution with a disposable injection needle to solidify, forming white solid particles. The obtained solid particles are dehydrated by gradient alcohol (dehydration steps: 80%, 90%, 95%, 100% ethanol with various concentrations are dehydrated for 2 hours respectively), transparent in xylene, soaked in paraffin, embedded in paraffin into paraffin specimens.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com