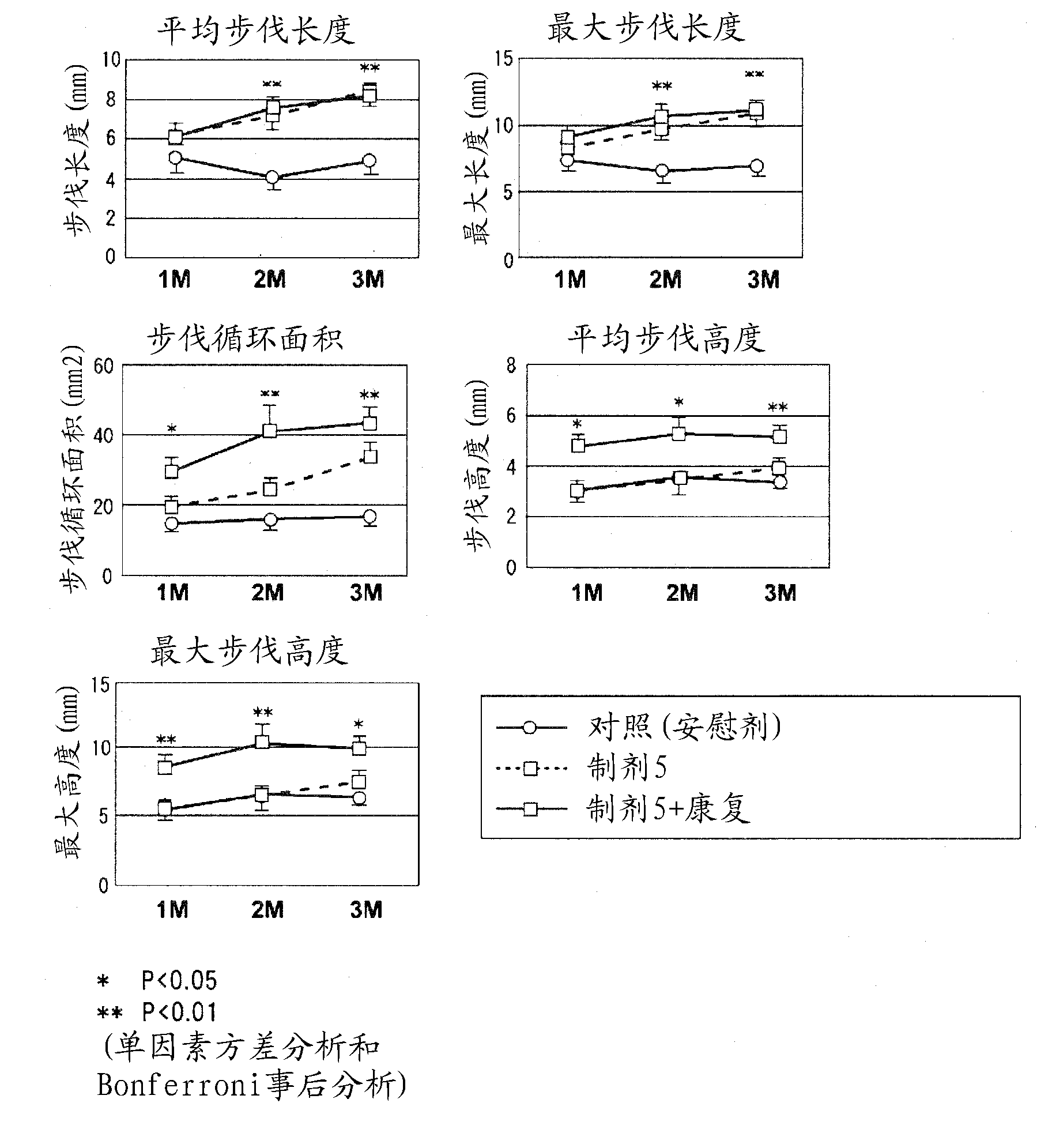
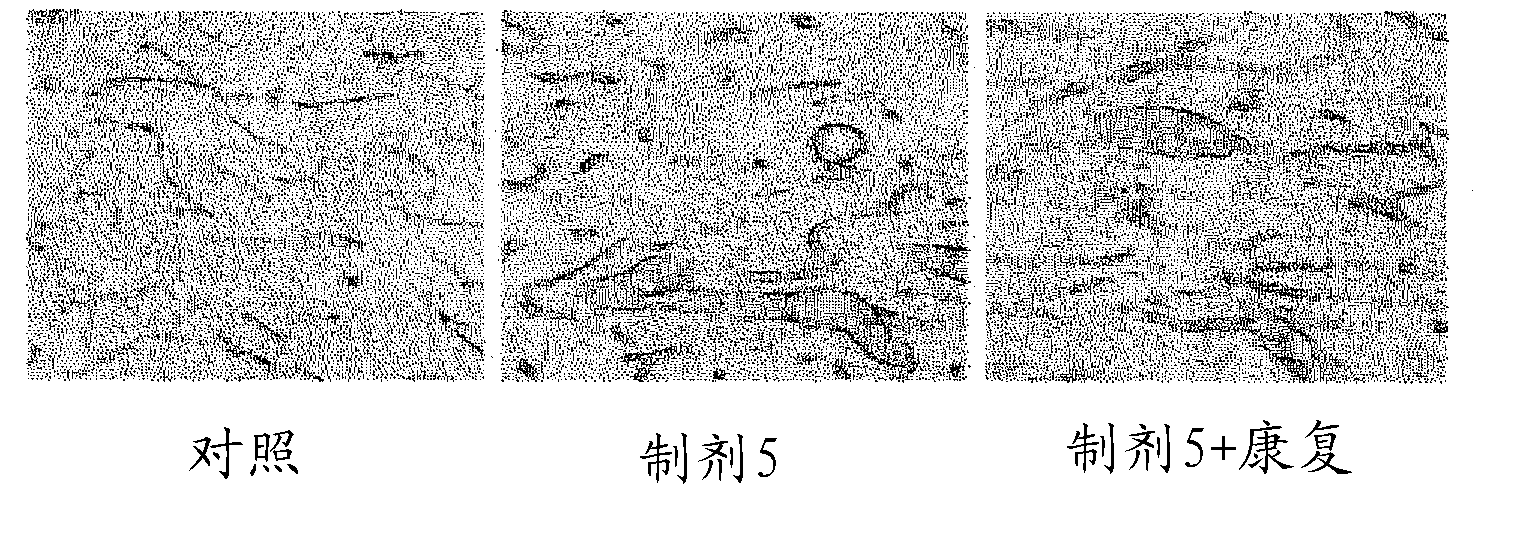
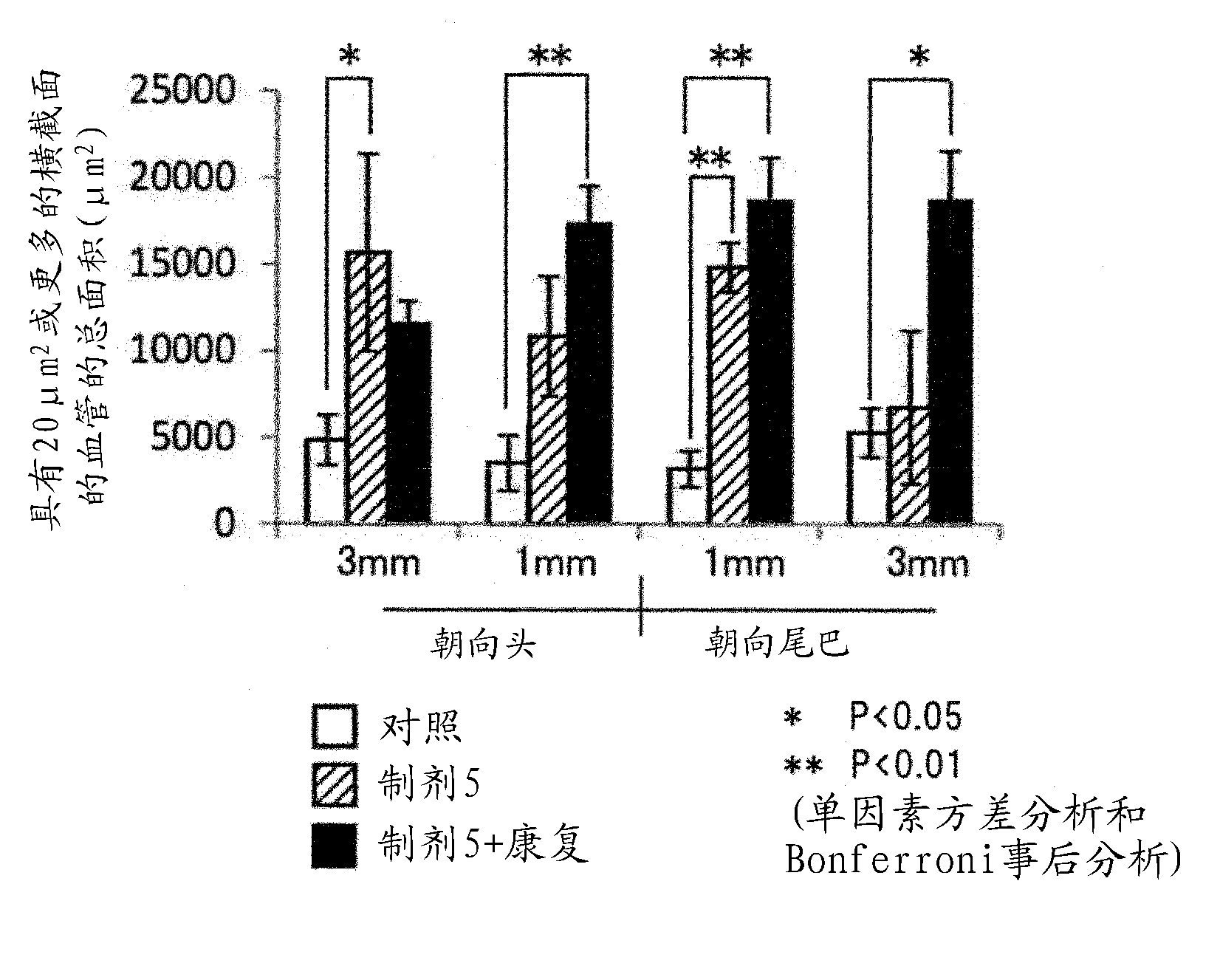Preparation for treatment of spinal cord injury
A technology of preparation and sustained-release preparation, applied in the field of medicine for local treatment of spinal cord injury
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0145] L-HPC (low substituted hydroxypropyl cellulose) (90 mg) and vinaxanthone powder (6 mg) were mixed in a mortar to produce a mixed powder. SILASTIC Q7-4750 Silicone A Component (102 mg) and Silicone B Component (102 mg) manufactured by Dow Corning were kneaded together using a double roller. After kneading the above-mentioned silicone, all of the above-obtained mixed powders were quickly added thereto, and the mixture was kneaded. Then, the kneaded product was rolled into a sheet shape using a double roller and cured at 40° C. for 1 day to yield a sheet preparation with a thickness of 0.3 mm. The sheet formulation was cut to produce Formulation 1.
Embodiment 2
[0152] To lactose (45 mg) was added L-HPC (45 mg) followed by vinaxanthone powder (6 mg) in a mortar and all three powders were mixed together to produce a mixed powder. SILASTIC Q7-4750 silicone A component (102 mg) and silicone B component (102 mg) manufactured by Dow Corning were kneaded together using a double roller. After kneading the above-mentioned silicone, all of the above-obtained mixed powders were quickly added thereto, and the mixture was kneaded. Then, the kneaded product was rolled into a sheet shape using a double roller and cured at 40° C. for 1 day to yield a sheet preparation with a thickness of 0.3 mm. The sheet formulation was cut to produce Formulation 2.
Embodiment 3
[0154] Crystalline sodium chloride was ground in a mortar to adjust its particle diameter to 100 μm or less, wherein the particle size was checked using an optical microscope (phase contrast microscope BX-51-33-PHU-D, OLYMPAS). To the sodium chloride (15 mg) obtained above was added L-HPC (75 mg) and then vinaxanthone powder (6 mg), and all three powders were mixed together in a mortar to produce a mixed powder. SILASTIC Q7-4750 silicone A component (102 mg) and silicone B component (102 mg) manufactured by Dow Corning were kneaded together using a double roller. After kneading the above-mentioned silicone, all of the above-obtained mixed powders were quickly added thereto, and the mixture was kneaded. Then, the kneaded product was rolled into a sheet shape using a double roller and cured at 40° C. for 1 day to yield a sheet preparation with a thickness of 0.3 mm. The sheet formulation was cut to produce Formulation 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com