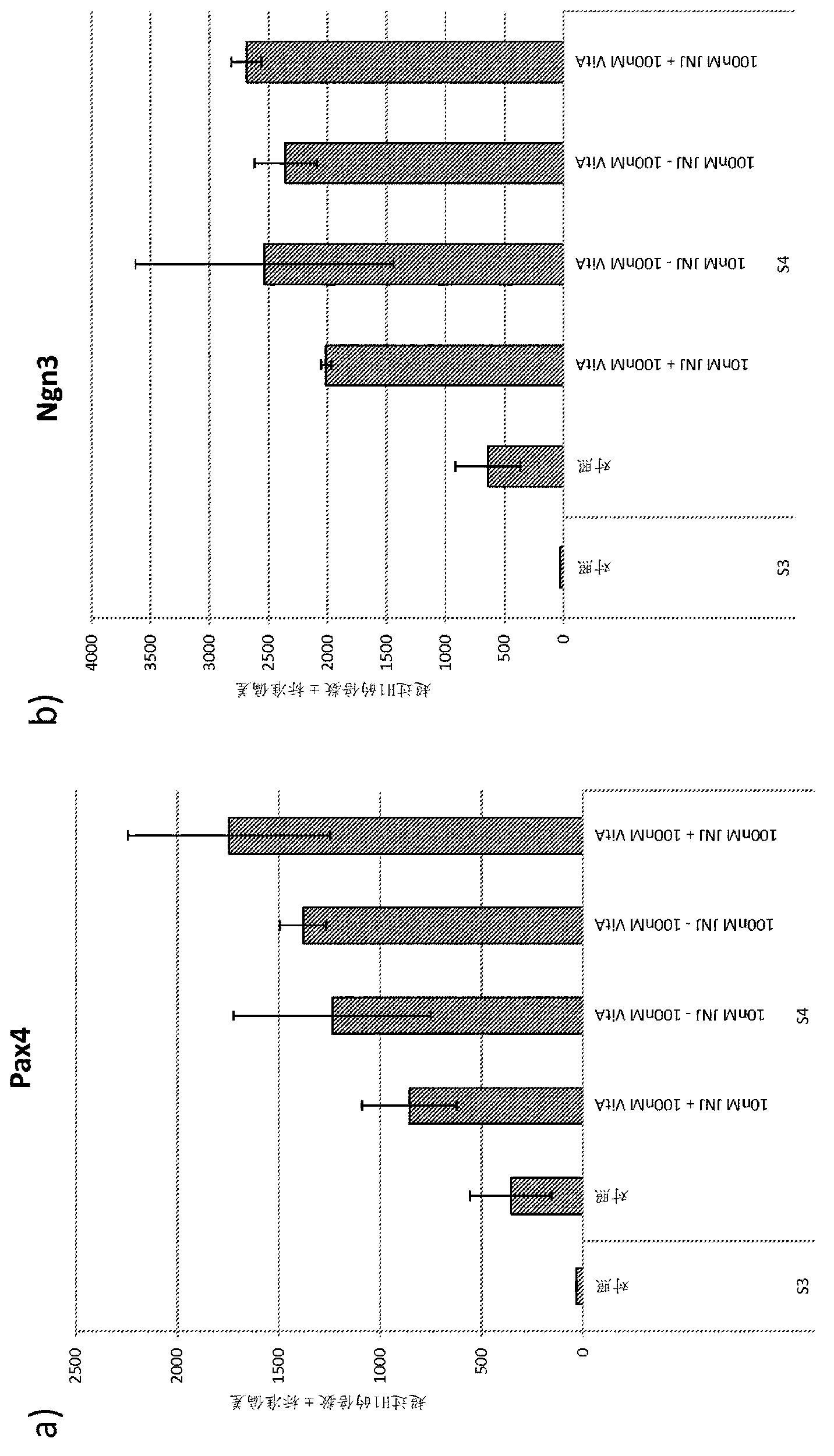
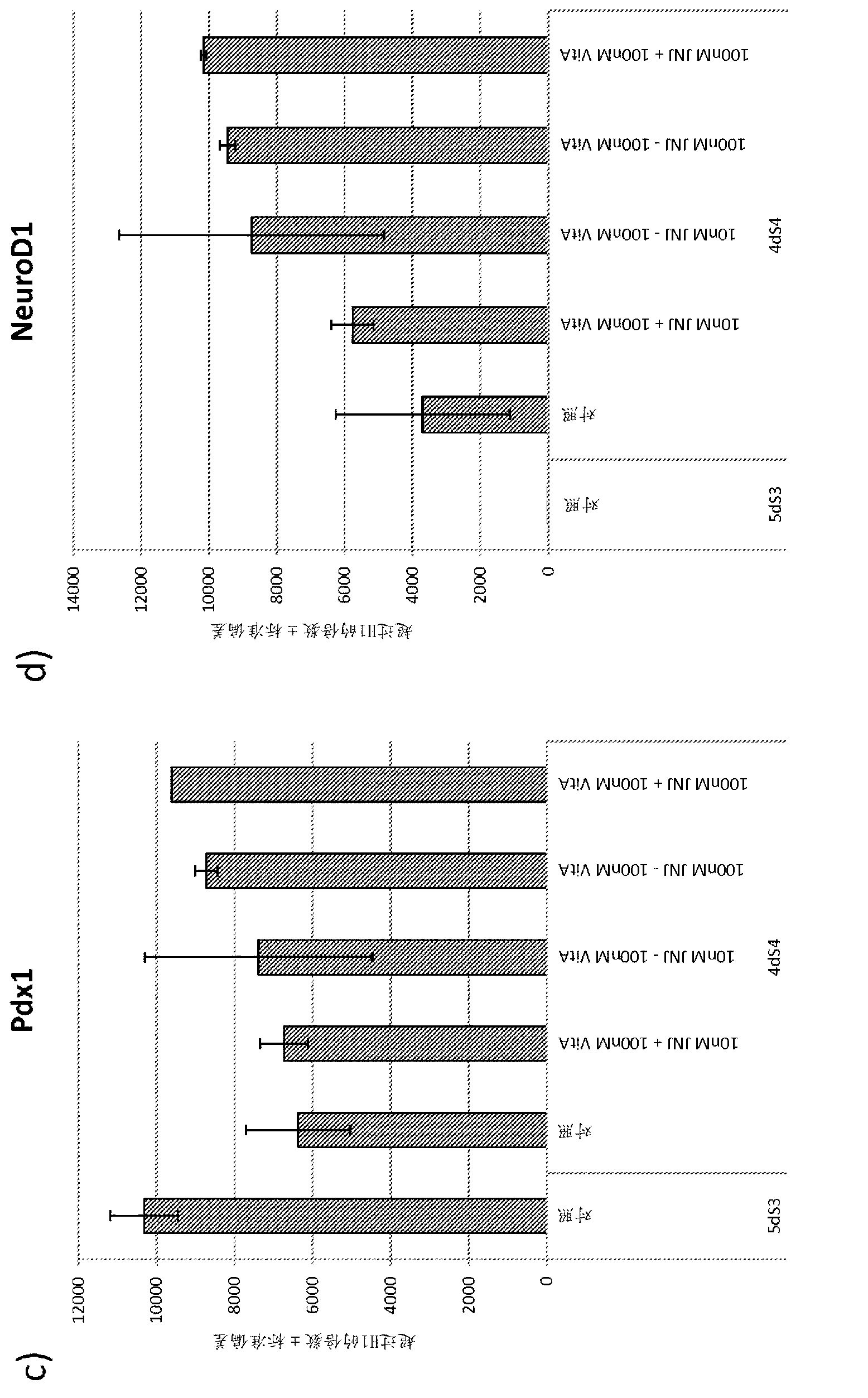
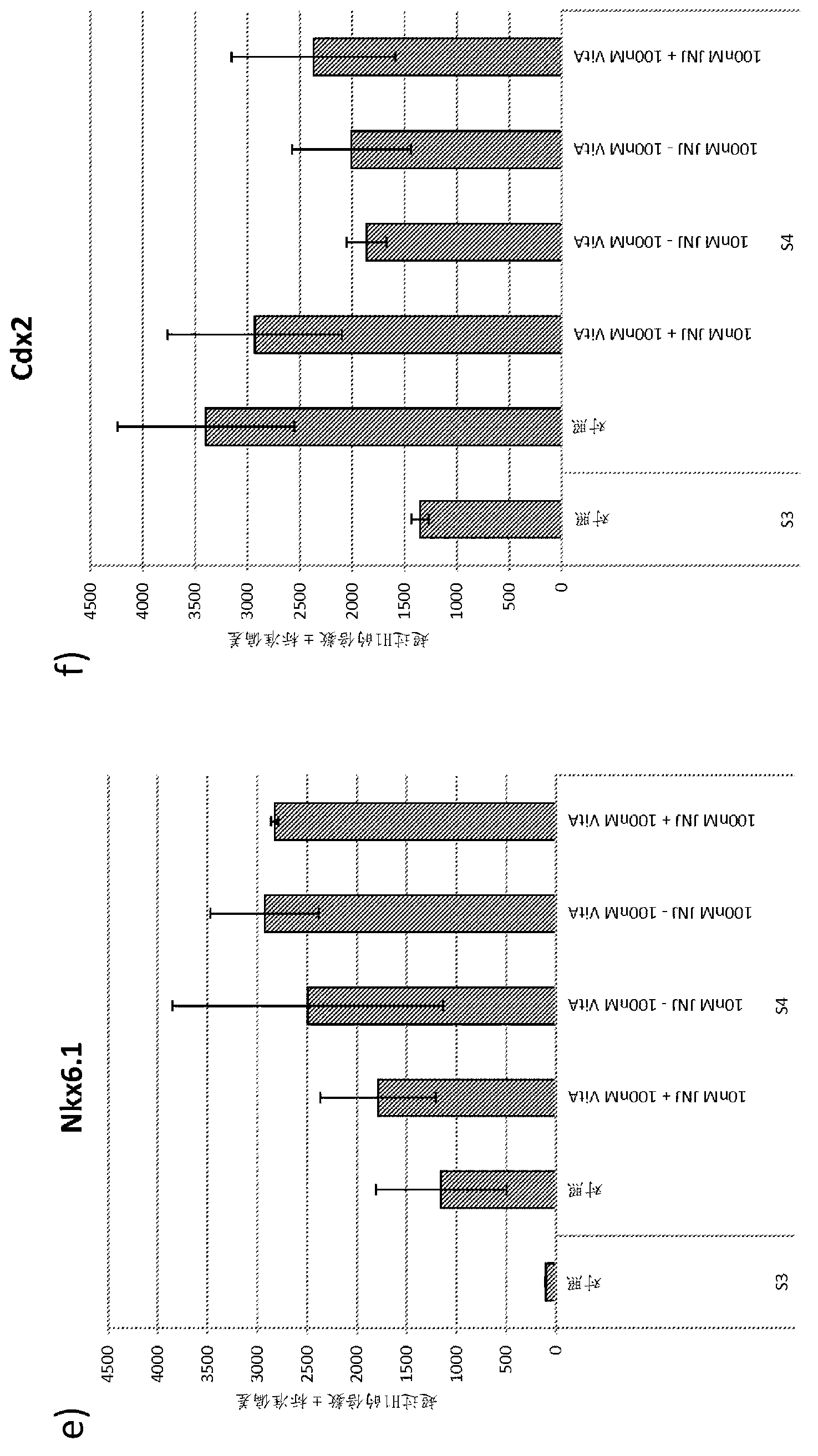
Differentiation of pluripotent stem cells
A technology of pluripotent stem cells and precursor cells, applied in the field of differentiation of pluripotent stem cells, can solve problems such as not completely simulating the developmental program of higher mammals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0121] Cells of the human embryonic stem cell line H1 were inactivated in cells lacking FBS and containing CYP26A inhibitors pancreatic endocrine progenitor cells
[0122] Cells of the human embryonic stem cell line H1(p40-p50) were colonized as Cultured in MEF-CM (Mouse Embryo Fibroblast Conditioned Medium) on coated dishes (1:30 dilution) (BD Biosciences; Cat# 356231 ) and differentiated into pancreatic endocrine precursor cells as follows:
[0123] a. Stage I (definitive endoderm): Human embryonic stem cell line cells were supplemented with 2% fatty acid-free BSA (Catalog No. 68700, Proliant, Iowa (Proliant, IA)) and 100 ng / ml Activin A (R&D Systems, MN) plus 20ng / ml WNT-3a (catalogue number 1324-WN-002, (Andy Biotech, Minnesota)) plus 8ng / ml bFGF ( Cat. No. 100-18B, PeproTech, NJ) in RPMI medium for one day and then re-treated with RPMI medium supplemented with 2% BSA and 100 ng / ml Activin A plus 8 ng / ml bFGF two days, then
example 2
[0129] Cells of the human embryonic stem cell line H1 were inactivated in cells lacking FBS and containing CYP26A inhibitors Alternative methods for differentiation into pancreatic endocrine precursor cells in culture
[0130] Human embryonic stem cell line H1 (p40-p52) cells were used as single cells at 100,000 cells / cm 2 planted at a density of Coated dishes (1:30 dilution) (BD Biosciences; Cat. No. 356231 ) were supplemented with 16 ng / ml FGF2 (Cat. No. 100-18B, PeproTech, NJ) and 10 μM Y-27632 (Rock Inhibitor, Cat. No. Y0503, Sigma, MO) in MEF-CM (Mouse Embryo Fibroblast Conditioned Medium). 72 hours after seeding, cultures were differentiated into definitive endoderm (DE) as follows:
[0131] a. Stage I (definitive endoderm): human embryonic stem cell line cells were supplemented with 2% fatty acid-free BSA (Cat. No. 68700, Proliant, Iowa), 0.0025 g / ml sodium bicarbonate (Cat. , Sigma Corporation of Missouri), 1X GlutaMax TM (Cat. No. 35050-079, Invitrogen, Calif...
example 3
[0138] Cells of the human embryonic stem cell line H1 were inactivated in cells lacking FBS and containing CYP26A inhibitors Alternative methods for differentiation into pancreatic endocrine cells in culture
[0139] Human embryonic stem cell line H1 (p40-p52) cells were used as single cells at 100,000 cells / cm 2 planted at a density of Coated dishes (1:30 dilution) (BD Biosciences; Cat. No. 356231 ) were supplemented with 16 ng / ml FGF2 (Cat. No. 100-18B, PeproTech, NJ) and 10 μM Y-27632 (Rock Inhibitor, Cat. No. Y0503, Sigma, MO) in MEF-CM (Mouse Embryo Fibroblast Conditioned Medium). 72 hours after seeding, cultures were differentiated into definitive endoderm (DE) as follows:
[0140] a. Stage I (definitive endoderm): Human embryonic stem cell line cells cultured as single cells on Matrigel-coated dishes were treated with supplemented with 2% fatty acid-free BSA (Catalog No. 68700, Proliant, Iowa) , 0.0025 g / ml Sodium Bicarbonate (Cat. No. S3187, Sigma, Missouri), 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com